Epilepsy Insights
Gene therapy for Dravet syndrome – 2020 update
There are multiple gene therapy and oligonucleotide programs in development for Dravet syndrome including those that supply and extra copy of the SCN1A gene and those that boost expression from the healthy SCN1A gene copy. Clinical trials have already started, with Stoke Therapeutics initiating the first clinical trial with a disease-targeting therapy in Dravet syndrome in summer 2020. Behind Stoke, gene therapies are approaching the clinic with Encoded Therapeutics having the most advanced clinical candidate and preparing for trials in 2021.
In February of 2019 I reviewed the state of development of gene therapy approaches for Dravet syndrome at that time. A lot has changed in a year a half, for good. So I felt this update was long due.
Here is a review of the gene therapies in development for treating Dravet syndrome, how each of them works, and the timings that we anticipate for clinical trials.
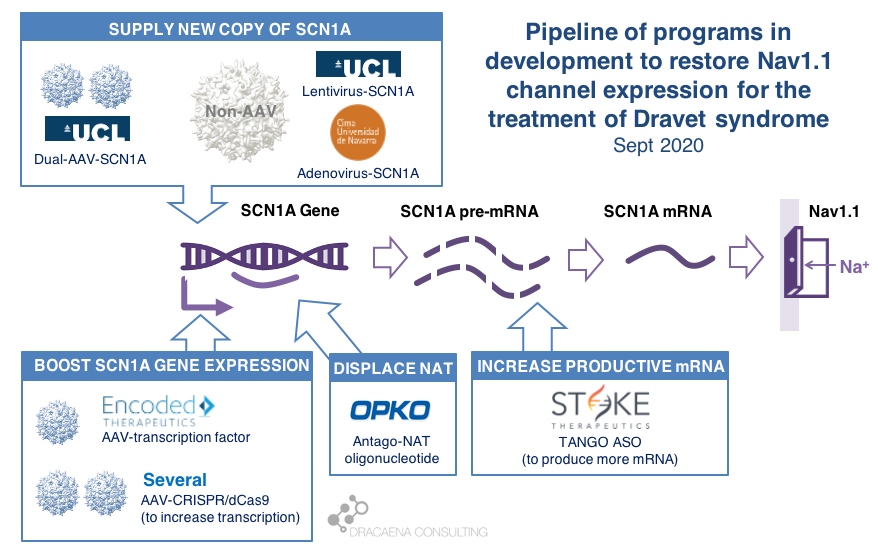
CURRENT GENE THERAPIES IN DEVELOPMENT FOR DRAVET SYNDROME
In diseases like Dravet syndrome where the problem is that a copy of the gene is missing or not functional due to mutations, the desired therapy is one that can restore normal gene expression and therefore normal protein production. In other words, we need more protein.
In the case of Dravet syndrome, the gene is SCN1A, and the protein that is needed is the neuronal sodium channel Nav1.1. As a result of mutations in the gene, the number of Nav1.1 channels at the neuronal surface is not sufficient, there is less sodium crossing the membrane, and the neuron cannot fire properly. The result is Dravet syndrome.
One particularity of Dravet syndrome is that only one of the two copies of the SCN1A gene is affected, the second one is perfectly fine, so that second copy can serve as the supply for extra protein production. As you will see, the most advanced programs are exploiting this possibility.
Broadly speaking, there are two potential approaches to restore protein expression in Dravet syndrome: you either supply the cell with an extra healthy copy the gene, which will lead to more protein being produced, or you try to boost the expression from the healthy gene.
(1) Supply a new copy of SCN1A
When people think about “gene therapy”, the type of therapy they are thinking about is the one where the DNA of a virus gets replaced by the gene that the person needs, and that modified virus is used as a Trojan horse to infect cells and deliver them the therapeutic gene.
The most commonly used virus for gene therapy is the Adeno-Associated Virus (AAV), and because we have so much experience with gene therapies being developed and approved using AAV, this virus is the first choice for most in the gene therapy space. But AAVs are small virus, and have a limited capacity for the size of genes that they can carry, and SCN1A happens to be way too large for using these virus to carry the gene. In January of 2018, before we knew about the new gene therapies currently in development for Dravet syndrome, I reviewed this problem in the article “big gene, small virus”.
One way around this is to use other viruses that are larger and can therefore carry larger genes inside. One of these is the Adenovirus, which we all hear much about recently because it is also the virus of choice for the AztraZeneca vaccine in development for COVID-19.
|| The Spain-France-Israel consortium CureDravet is developing a gene therapy for Dravet syndrome using Adenovirus, a type of high-capacity virus that is large enough to contain the entire SCN1A gene. The leading Spanish group from Rubén Hernández has presented early results at conferences (e.g. European Paediatric Neurology Society (EPNS) 2019) showing rescue of multiple disease phenotypes in transgenic mice by administration of an adenovirus-based SCN1A gene therapy. As of the last public update this is still a preclinical program working towards the development of a clinical candidate.
Another way around the gene-vs-virus size issue is to use not one, but two AAVs, and to make each carry half of the SCN1A gene. This is a strategy that is also being explored for gene editing using CRISPR because the CRISPR approach requires expressing in the tissue proteins whose genes are too large to fit into an AAV. So the strategy of two AAVs is being explored both for rare genetic diseases with large genes such as Dravet syndrome, and for gene editing in general.
|| At UCL, the team of Rajvinder Karda and Simon Waddington is working on two approaches. One is to use another type of large-capacity virus, Lentivirus, to carry the SCN1A gene. The second approach uses two AAV virus, each containing half of the SCN1A gene, which are able to recreate the full channel once they co-infect the same cells. As of the last public updates these are also still preclinical programs working towards the development of clinical candidates.
(2) Boost expression of SCN1A
Another strategy to restore Nav1.1 levels is to target the good SCN1A gene copy or some stem downstream of it to increase protein production, without needing to add an external gene copy with a virus. This one is the strategy most advanced for Dravet syndrome.
There are several ways to do this, and luckily for us many of these approaches are being pursued and some of them are already in clinical development or getting very close.
As a small biology primer: genes (like SCN1A) are large stretches of DNA that contain the information needed to produce proteins (like Nav1.1). These large stretches of DNA are first copied into large stretches of RNA (pre-messenger RNA or pre-mRNA), then some sections that are not meant to be part of the final protein are removed from the RNA to make it shorter (mRNA), and then the mRNA is read to produce the final Nav1.1 protein.
What happens in Dravet syndrome as a result of having a mutation (or deletion) in one copy of the SCN1A is the following:
By now we know that the levels of Nav1.1 protein production are regulated in the cell at different levels along this sequence of steps, offering scientists multiple points where to act when trying to boost production of Nav1.1:
Regulation at the step of gene expression 1: the SCN1A gene has sequences around it that tell the cell how much the gene should be copied and therefore how much RNA should be produced. These are promoter or enhancer or other regulatory regions that could be targeted to boost gene expression and protein production.
Regulation at the step of gene expression 2: cells produce some RNAs that are not meant to produce proteins, but to match the sequence of some genes and prevent them from being read by the cell. SCN1A happens to be the target of one of those Natural Antisense Transcripts, or NAT. This endogenous “repressor” of SCN1A expression could be also targeted to boost gene expression and protein production.
Regulation at the step of RNA processing: neurons produce a large amount of pre-mRNA of SCN1A, with only a fraction of it being processed into mRNA which is the final form that will be used to produce proteins. This creates a “reserve pool” of pre-mRNA that could potentially also be targeted to get more productive mature mRNA and therefore boost protein production.
The first company to try one of these approaches was OPKO Health. OPKO decided to go after the Natural Antisense Transcripts that limits SCN1A expression by developing an oligonucleotide that could displace it, releasing the good SCN1A copy and leading to more mRNA and more Nav1.1 protein levels. Because their oligonucleotide antagonizes the NAT for SCN1A they called it an AntagoNAT (OPK88001, previously CUR-1916). While OPKO indicated plans to initiate clinical trials as early as in 2017, these timelines were moved to 2018 and then 2019 and as of summer 2020 there are no news of whether this program is still active. In the meantime, other corporate programs have appeared and progressed further into the clinic.
As a side note: oligonucleotide therapeutics are NOT gene therapies, if we stick to the actual meaning of gene therapies using genes as therapies. But because oligonucleotide therapeutics target gene expression or mRNA processing leading to more protein production we often bundle them together with the “real gene therapies”. Both are approaches that target the genetic problem in the disease, either correcting it or compensating for it (like when boosting the healthy gene copy expression). Both are expected to result in increased levels of Nav1.1. And both are expected to lead to transformational improvements across different symptom domains in patients. So for all purposes it makes sense to talk about together when reviewing gene therapies.
2018 brought the good news that Stoke Therapeutics was developing an antisense oligonucleotide treatment to boost expression of SCN1A as well. This oligonucleotide binds to the pre-mRNA, facilitating the transformation of some of the pre-mRNA “reserve pool” into mature mRNA and leading to more Nav1.1 protein. Stoke recently published their preclinical proof of concept (July 2020 and August 2020), showing how they were able to produce an antisense oligonucleotide able to trigger this pre-mRNA to mRNA processing specifically for SCN1A and not affecting other sequence-related sodium channel genes. Their therapeutic candidate, called STK-001, increased expression of the SCN1A mRNA and Nav1.1 protein in mice, and had remarkable efficacy in mice with Dravet syndrome caused by SCN1A haploinsufficiency.
|| Since my early 2019 update, Stoke Therapeutics had an IPO, obtained an FDA Orphan Drug Designation for STK-001, and in August of 2020 it initiated a Phase 1/2s study in patients 2 to 18 years with Dravet syndrome. The dosing of the first patient in this study represented a historical moment for the Dravet syndrome field: for the first time, a person born with Dravet syndrome was given a therapy designed to correct their genetic problem (SCN1A haploinsufficiency). That day the field moved from developing and testing symptomatic treatments to developing and testing disease-targeting treatments, which the patient community often prefers to call cures.
The program from Stoke Therapeutics is the most advanced of all gene therapies and gene therapy-like approaches in development for Dravet syndrome. The second program in line is a gene therapy approach by Encoded Therapeutics that was still in stealth mode in 2018.
|| In June 2019, Encoded Therapeutics emerged from stealth mode with a series C of $104M with a lead program for Dravet syndrome. Encoded managed to beat the gene-vs-virus size problem by using AAV, the gold-standard viral vector for gene therapy, to deliver to the brain a synthetic transcription factor for SCN1A. In other words, the SCN1A gene might be too large, but we can still AAV to deliver to the brain the gene of a specific SCN1A booster. In barely one year, Encoded has closed another funding round of $135M, obtained the Orphan Drug Designation and Rare Pediatric Disease Designation by the FDA for their ETX101 gene therapy for Dravet syndrome, and anticipates to start clinical trials in 2021.
Several academic efforts follow Stoke and Encoded in the pursue of boosting expression from the good SCN1A gene copy. All of these projects are in early preclinical stages, and they have not yet published a solid proof of concept in a Dravet syndrome mouse model, which is an initial stage prior to advancing the treatments towards clinical trials. These programs are therefore all years away from clinical trial initiation, with no guarantee of succeeding.
|| An academic group in Italy, with funding from CURE and the Dravet Syndrome European Federation, is researching an oligonucleotide approach to boost production of Nav1.1 protein though a combination of transcriptional and translational activity. There have been no recent public updates on the progress of this project.
|| The Vania Broccoli lab in Italy in collaboration with Gabriele Lignani lab at UCL are using a CRISPR approach to target the promoter of SCN1A and increase its activity, therefore boosting production of Nav1.1 from the good SCN1A gene copy. They use a variant of CRISPR that uses dead Cas9 to find – but not to cut – the desired genomic region and activate it. They have demonstrated the ability of upregulating Nav1.1 using this system using lentivirus in vitro, and published an early proof of principle in mice by using two AAVs to deliver all of the different elements of the CRISPR system into the brain, which would not otherwise fit into one AAV. It is early to know if these dual-AAV approaches will achieve sufficient biodistribution and expression in the brain, or if single-AAV or single-Adenovirus approaches are needed, probably reducing the size of the cargo like Encoded is doing with their transcription factor approach.
|| The Yamakawa lab in Japan has also published a recent study using CRISPR with dead Cas9 to increase SCN1A gene expression and protein production in mice. For multiple reasons, their approach cannot be considered a proof of concept for the future clinical treatment. For example they used a type of AAV (PHP.B), which has fantastic biodistribution in mice but not in primates, and administered it via intravenous administration. Peripheral administration of AAV to target neurological diseases also seems an approach less viable in patients than in mice. In this case part of the CRISPR system was genetically encoded in the mice, so they only needed administration of one AAV. This would also not be possible in patients, who would need the entire treatment to be delivered to them using suitable route of administration and virus type. But what this study provides is a proof of biologic rescue of SCN1A expression in GABAergic neurons starting at 4 weeks of age in mice, and the improvement in seizures, mortality and behavioral phenotypes that result from such rescue.
|| SCN1A encodes for the alpha subunit of Nav1.1, so less alpha subunit means less mature sodium channels, but there is also a beta subunit involved in channel formation which is encoded by SCN1B. The Hampson lab in Toronto recently published a gene therapy approach using AAV to deliver an additional copy of SCN1B, which is a small gene that fits the virus. The reasoning is that upregulating the beta subunit might be able to drag more alpha subunits to the surface and result in more total Nav1.1. In the study, funded by Dravet Canada and the US Dravet Syndrome Foundation, mice heterozygous for SCN1A had improvements in mortality, seizures and behavioral outcomes, although some of these were more pronounced in males or females. None of the SCN1A boosting approaches had reported any sex-bias when it comes to efficacy. The beta subunit produced by SCN1B is auxiliary to multiple channels, not unique to Nav1.1, and the study did not determine which of these channels might be mediating efficacy, so the relevance of this study to the SCN1A-targeted therapeutics is unclear.
FINAL THOUGHTS
There are several differences between oligonucleotide treatments and gene therapies. Some of these are differences that are more important for us scientists. For example to better understand the need to increase SCN1A expression in all cells that naturally express the gene (what oligonucleotides do) versus the convenience of boosting it only in those that are the most affected in the disease (an option available only for gene therapies because they can use cell-specific promoters). Other differences are more important to patient families, such as the need to repeat dosing with oligonucleotide treatments, usually spaced out by several months, versus the once-and-done single-administration approach of the gene therapies. In the end what we all want to see is which approach produces the most clinical improvement with the least side effects and burden of administration, and it is very likely that we will want to have multiple options in the market.
When thinking about these options, the field of SMA comes to mind. Spinraza (by Biogen) is an ASO able to increase the production of SMN, and a total game-changer in a terrible disease that used to kill babies. Then Zolgensma (by Novartis) reached the market, as the first gene therapy using AAV to deliver a copy of the SMN1 gene. With both in the market we have started seeing clinical studies to evaluate the combination of the ASO and the gene therapy in patients with SMA. And last month FDA approved risdiplam (Evrysdi) from Genentech/Roche which is a small molecule that can be taken orally and that increases the production of SMN. In 4 years the field of SMA has gone from facing a death sentence to having three very different modalities all able to rescue the missing protein expression (ensuring access to these treatments is a different topic, but I still want to highlight that there is a lot of work to do there).
So while we keep an eye on Stoke and Encoded and the development of their two very different approaches, we might want to keep another eye following the developing of small molecule activators of the Nav1.1 channel, for which there are several programs in the development.
We also want to make sure that the current oligonucleotide treatments and gene therapies in development are successful. A major challenge they face is that we don’t know how to design a clinical trial with a gene therapy in Dravet syndrome. So far clinical trials measuring seizure frequency have been very successful, but a gene therapy is expected to improve the syndrome beyond just seizure frequency. The field of Dravet syndrome is still immature when it comes to clinical outcome measure development and validation for non-seizure outcomes (for non-scientists in the audience: we don’t know how to quantify improvements of the disease in a clinical trial beyond seizures). Both Stoke and Encoded are running observational studies that will hopefully identify the best outcome measures and endpoints that will be needed for pivotal studies.
And another development that I miss is that despite so many approaches trying to increase the levels of Nav1.1 we don’t have any biomarker that could help us measure the levels of functional or total Nav1.1 in patients. This will make it hard to interpret clinical trials where a dose of the treatment is ineffective based on the selected outcome measures. And having a biomarker for protein levels will provide early data to encourage longer patient monitoring as we wait for some of those outcome measures to show enough of a change. So in parallel to a race for developing new treatments, we are seeing a race to de-risk the field fast enough so that those treatments get their best shot at succeeding in a pivotal trial.
IN SUMMARY
There are multiple gene therapy and oligonucleotide programs in development for Dravet syndrome including those that supply and extra copy of the SCN1A gene and those that boost expression from the healthy SCN1A gene copy.
Clinical trials have already started, with Stoke Therapeutics initiating the first clinical trial with a disease-targeting therapy in Dravet syndrome in summer 2020.
Behind Stoke, gene therapies are approaching the clinic with Encoded Therapeutics having the most advanced clinical candidate and preparing for trials in 2021.
I look forward to seeing precompetitive collaborations around the common challenges of validating clinical outcome measures and biomarkers, which are needed to maximize the success of gene therapies for Dravet syndrome.
Ana Mingorance PhD
2016 numbers: CNS orphan drugs growing
With 2016 numbers now available, the number of orphan drugs in development for neurological indications is looking quite positive. I have reviewed the numbers of orphan drug designations and approvals by FDA in 2016 to see how popular are neurological orphan drugs today and what the trend is for the near future.
With 2016 numbers now available, the number of orphan drugs in development for neurological indications is looking quite positive. I have reviewed the numbers of orphan drug designations and approvals by FDA in 2016 to see how popular are neurological orphan drugs today and what the trend is for the near future.
The FDA approved only 22 new drugs last year, a big fall from the 45 approvals it granted in 2015. But not all approvals are for new molecules, there are also many approvals that are new indications of previously approved drugs, also known as “drug repurposing”, so the total number of approvals is actually higher than what news outlets otherwise suggest (see here and here).
We see many cases of drug repurposing in rare diseases, so I have looked at the total number of orphan drug approvals and the total number of orphan drug designations in 2016, including new molecules as well as new indications (or designations) for already approved drugs.
THE NUMBERS
In 2016 the FDA approved 37 orphan drugs, out of which only two were for neurological indications: Spinraza (nusinersen) from Biogen for spinal muscular atrophy, and Carnexiv (carbamazepine injection) from Lundbeck for epilepsy as an alternative to oral carbamazepine. The rest of the approvals, as usual, were dominated by oncology.
This means that only 5% of all orphan drug approvals during 2016 were for neurological indications.
However when we look at the drugs that received an orphan drug designation last year the picture is much better for neurology.
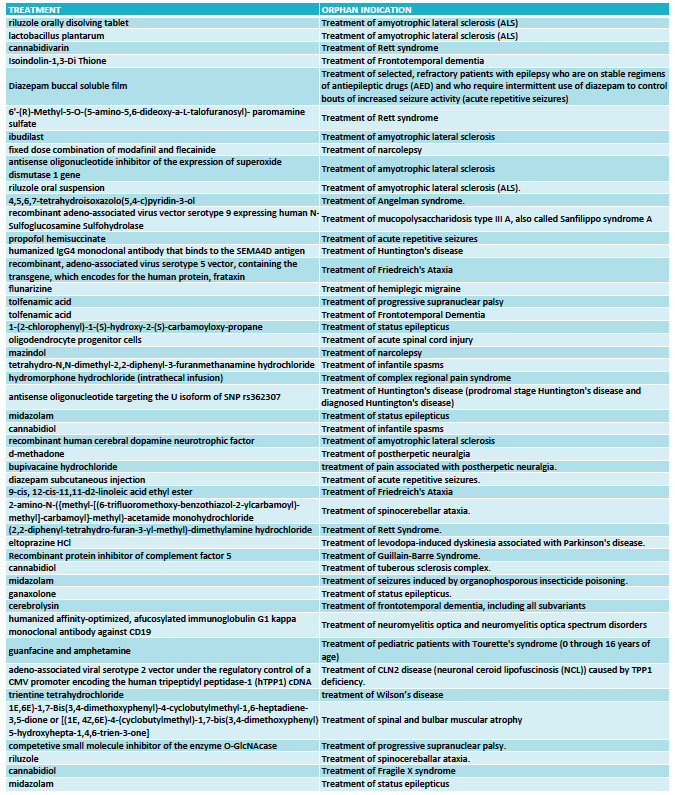
The FDA granted 333 orphan drug designations last year. This number includes some diagnostic reagents so I have only included in my analysis 325 orphan drug designations for the treatment of rare indications. Out of these, 48 orphan drug designations were for neurological indications (see full list at the end of the article) and again oncology dominated in the remaining cases.
With 48 designations in 2016, neurology accounted for 15% of the cases, indicating that the percentage of orphan drugs approvals for neurological indications could triple in the near future.
THE BREAKDOWN
Among the 48 designations, the largest area was neurodegeneration, followed by epilepsy. This reflects two important trends in CNS drug development.
One trend is to target orphan indications when the large indications become too crowded. This is the case of epilepsy, where many of the orphan designations are for drugs that could have efficacy in broader epilepsies -and in many cases are already approved for broader forms of epilepsy- but choose to seek approval for a specific syndrome or type of seizures (such as acute repetitive seizures) to reduce competition.
The other trend is to use rare diseases to obtain the initial clinical proof-of-concept for a compound that is eventually aimed at targeting the large indications. This has became very common in the neurodegeneration field where a Phase 3 failure can cost many hundreds of millions, so de-risking the program in a smaller and more uniform patient population is an excellent development strategy. Amyotrophic lateral sclerosis (ALS) was traditionally the rare disease model for neurodegenerative compounds and had 6 orphan drug designations in 2016, and frontotemporal dementia (FTD) and progressive supranuclear palsy (PSP) have emerged more recently as attractive rare diseases to re-risk molecules in development for Parkinson’s disease or Alzheimer’s disease.
What I classified as neurodevelopmental diseases includes the syndromes of Rett, Angelman, Fragile X and tuberous sclerosis complex. I could have classified some of these as epilepsy based on the trial endpoint, like is the case of the orphan drug designation for treating tuberous sclerosis complex with cannabidiol. Likewise, I could classify some of the epilepsy syndromes as neurodevelopmental diseases given the clinical presentation but kept them under epilepsies for this analysis purposes because of the seizure-focused trial endpoints. Collectively, all of these neurological syndromes characterized by seizures, cognitive, behavioral and motor problems are very popular within the orphan drug space.
Among the remaining designations I found interesting 4 treatments for ataxias (Friedreich's ataxia and spinocerebellar ataxia), and two for narcolepsy.
THE FUTURE
Looking at the 2016 orphan drug designations in neurology (see the full list at the end of the article) I can see how rare diseases are facilitating a revival of a field that has suffered from some areas being overcrowded and others being extremely difficult to pursue (think of Alzheimer’s disease). By providing smaller and more homogenous populations rare diseases offer the possibility of shorter and cheaper development programs, opening the field to smaller companies that could now have developed a clinical asset without partnering with a large company. And the genetic nature of many of the rare diseases has also offered us targets that increase the likelihood of succeeding in those trials versus symptomatic approaches in heterogeneous populations.
But not all is opportunistic strategies to reduce competition or de-risk programs. We are also seeing a growing number of gene therapy or antisense approaches in development that target the cause of these rare diseases, including 2016 designations for Sanfilippo syndrome, Friedreich's ataxia and Batten disease and antisense oligonucleotides for ALS and Huntington's disease.
Based on these numbers the future looks promising for neurological orphan drugs, with numbers that could triple and the development of disease-modifying treatments.
Ana Mingorance PhD
FULL LIST ORPHAN DRUG DESIGNATIONS FOR NEUROLOGY - FDA 2016
How close are we to creating transgenic people?
The journal Nature just released one of the most anticipated breaking news of the last few years: CRISPR gene editing has been tested in a person for the first time. In my day-to-day work I interact with families that have a child with a genetic disease. I get one question a lot: how close are we to turn that discovery into a therapy for people with genetic diseases?
The journal Nature just released one of the most anticipated breaking news of the last few years: CRISPR gene editing has been tested in a person for the first time.
In my day-to-day work I interact with families that have a child with a genetic disease. These are diseases where the mutation is produced “de novo”, which means that it happened during the production of the baby. The family didn’t carry any mutation and most likely that child is the only one with that exact mutation, or one of a handful world-wide.
As you can imagine, ever since scientists announced they had found a way to do copy-and-paste in DNA to introduce or to correct mutations I get one question a lot: how close are we to turn that discovery into a therapy for people with genetic diseases?
And despite the breaking news from Nature the answer is still “not that close”.
Let me elaborate on that.
Before being available as a treatment for people born with genetic mutations the CRISPR technology needs to go through roughly four steps:
Show that CRISPR can correct mutations in human cells in a test tube. This was the groundbreaking discovery that got us started in an amazing medical development explosion.
Show that these genetically-modified cells can be delivered to patients. This is more cell therapy than gene therapy and it is very useful on its own. Likely to be the first path forward for the CRISPR approach.
Show that we can actually correct mutation in patients using CRISP. Now we are talking about gene therapy, and for years this will have to be done in clinical trials under controlled conditions.
Finally, get approval for gene therapy using CRISPR technology so that we can fix patient’s mutations, effectively creating transgenic people.
What Nature announces is that scientists at Sichuan University have treated a patient with lung cancer with cells that had been reprogrammed using CRISPR before being delivered into the patient. This is step 2.
It had also been done before using other technologies that enable gene-editing, also in diseases where scientists first modify those cells outside the body and then deliver them to the patient, such as in cases of leukaemia or HIV. CRISPR is predicted to be the most powerful (and easy!) of these methods and is likely to be the one that will become a real treatment so reaching step 2 is great news.
At this second stage, these are all “ex vivo” approaches where the gene editing technology is applied to the cells that will be used to treat the patient, instead of using the technology directly in the patient.
The move towards step 3, editing the patient DNA, opens serious safety concerns:could the gene-editing enzymes cut and paste more letters in the DNA that they were intended to? Could they cause unwanted mutations? Because of that, it is likely to happen first in very localized indications such as tumours or retinal disease.
Moving from those localized diseases to more widespread ones will have the same challenges to reach the target organs that “traditional gene therapy” currently has. For the non-initiated, “traditional gene therapy” doesn’t change the patient DNA, instead it uses virus that have been stripped of the virus DNA to infect the patient and deliver a healthy version of the gene that the patients have mutated. At the end the patient has his own genes plus this new therapeutic gene. And that is not easy to do in hard-to-reach organs such as the brain!
Because delivering the CRISPR enzymes will also rely on viral vectors, even if CRISPR was proven today to be totally safe we wouldn’t know how to apply it to the brain tomorrow, since we still haven’t mastered that delivery aspect yet.
For any patient with a neurological condition caused by de novo mutations (so mutations unique to him/her) this is how the things to do list looks like before we can treat him:
CRISPR needs to be proven safe in small regions (step 3)
We need to find good viral vectors to deliver genes to the brain, which is larger than the eye or blood cells and happens to come inside of a hard box.
Those viral vectors also need to be safe.
CRISPR will be first used for genetic neurological diseases that are inherited, which means where the same mutation is found in many people.
And only after all that has happened we can think of using CRISPR therapy when the target mutation is unique for each patient, which introduces additional questions: can we get approval for the disease and just change the target sequence that the CRISPR uses? Will companies ever manufacture separate ones for individual patients? etc
So for the patients I work with, who carry de novo mutations that cause neurological diseases, the answer to how close we are to use CRISPR as a therapy for people like them can only be “not that close”. These are probably the last diseases to benefit from the gene editing technology.
In the mean time we celebrate that we have reached step 2 with CRISPR, delivering edited cells to a patient, and keep our eyes on that next frontier: the in vivo experiment, the first patient that has his own DNA corrected using CRISPR in a clinical trial, the first transgenic people.
Let me know what you think about it in the comments.
Ana Mingorance PhD
Originally published in LinkedIn on November 16, 2016