Epilepsy Insights
Clinical trials in CDKL5 Deficiency Disorder – 2Q 2019
There are currently 4 clinical trials ongoing or about to start in CDKL5 Deficiency Disorder: ataluren, ganaxolone, TAK-935 and fenfluramine. This article is a summary of where we are with clinical trials for CDKL5 Deficiency Disorder for families and other interested readers including what we know about these four drugs, their efficacy, at which level of clinical development they are at, and where can you learn more about these trials.
Some rare diseases can go for many years without much research on them. Other rare diseases, however, attract so much attention from scientists and companies that in a couple of years can make the progress that would have otherwise taken decades.
This is the case of CDKL5 Deficiency Disorder (CDD), a monogenetic rare disease that affects brain development and causes a very severe epilepsy with hundreds of seizures a month. I also reviewed all of the news on CDD in my review of the last CDKL5 Forum meeting HERE (October 2018).
This article is a summary of where we are with clinical trials for CDKL5 Deficiency Disorder for families and other interested readers.
FIRST, A NOTE ABOUT CLINICAL TRIALS
There are currently 4 clinical trials ongoing or about to start in CDD.
If you are coming from the patient side, and not from the medical community, you have probably heard about clinical trials being always divided in three stages:
Phase 1 trials: Small trial (study) in healthy adult volunteers, not in patients. The purpose of this phase is to determine the safety of the drug, as well as to explore different doses of the drug and measure the biodistribution (how soon you eliminate it, how it breaks down, does it accumulate… etc)
Phase 2 trials: Also known as “pilot” trials. Small trials in real patients with the disease that the drug intents to treat. This phase is mainly intended for determining safety, and also to pick a sign of efficacy. There are usually not enough patients to be sure that the drug works, but it enables companies and regulators to decide to move on to the next phase if the data looks good.
Phase 3 trials: Also known as “pivotal” trials. These are large confirmatory studies, in patients, with a placebo-controlled group (to serve as a control of what the normal change in the disease would have been in the absence of the drug, everything else remaining the same). You often need two of these trials for approval. Depending on the disease, it could be over a thousand patients per group. Other times it is many hundreds. Because of the need of finding so many patients it can take years to complete.
This is the default design, but there are exceptions to it. One exception is that when diseases are rare, you often need fewer patients, and might only need one pivotal trial to show efficacy and get a drug approved. This means a company can go from starting clinical trials to approval in 4 years, as GW Pharma recently did with Epidiolex for Dravet and Lennox-Gastaut syndromes, as opposed to 10+ years on non-orphan diseases!
Another exception is when the drug being tested has already gone through a Phase 1 evaluation in the past when being considered for other diseases, so once the company that owns the drug shows an interest in your disease they can move straight into Phase 2 (pilot) trials.
Both exceptions are true for CDD, so as you will see below our timelines are much faster than the usual length of Phase 1 + Phase 2 + Phase 3 trials that you will find described in most on-line materials. Also, our pilot (Phase 2) trials need less than 20 patients, and the first drug that has reached the pivotal (Phase 3) stage only needs one trial with 70 patients. This makes it all a bit more doable and a lot faster.
One important note about CDD trials is that all of them add the experimental drug or the placebo treatment to the other medications that the patient is already taking for a duration of 12 to 17 weeks. This means that no participant will ever find themselves in a “no treatment” trial group if receiving placebo, they will simply start adding the actual experimental treatment to their usual medications later. Some of the clinical trials do not even include placebo group.
So let’s jump into the update: which are these four drugs, what do we know about their efficacy, and where can you learn more about these trials or what comes after them.
CDKL5 DEFICIENCY DISORDER CLINICAL TRIALS REVIEW
From the drug that first started clinical trials in CDD to the one that is about to start, these are the four clinical trials for CDD that you should know about:
#1 ATALUREN – PTC THERAPEUTICS
Ataluren is a drug approved in Europe for the treatment of a subset of patients with Duchenne Muscular Dystrophy and marketed under the name of Translarna. It is currently completing a placebo-controlled Phase 2 (pilot) study at NYU Langone Medical Center in children with CDKL5 Deficiency Disorder caused by non-sense mutations.
How does this drug work?
The brand name of ataluren is “Translarna” because it facilitates translationof RNA. I like how clever the name is. What it does is to target specifically a type of mutations known as non-sense mutations, which cause a premature stop in the gene sequence. These mutations appear in many different genes. That is why we can test this drug in diseases other than Duchenne, such as CDD and Dravet syndrome (the second disease evaluated in the same clinical trial). What ataluren does is to make the cell read through that premature stop and complete the protein that is otherwise missing in that disease.
Is there any previous clinical experience with this drug?
Yes! Ataluren is already approved for Duchenne, so the active dose and the safety profile are known. There is still no efficacy data in patients with neurodevelopmental diseases or epilepsy because these CDD and Dravet syndrome trials are still ongoing. One important difference with Duchenne is that CDD and Dravet are diseases of the brain, not the muscles, so it is possible that the drug doesn’t get well-enough into the neurons to work in these neurodevelopmental diseases. We will have to wait for the trial results to know that.
How is the clinical trial in CDD?
The trial is a pilot study, involving 9 patients with CDD that all go through some months in treatment and some months in placebo. This is called a “crossover” design, where some patients start in the drug and are then crossed over to placebo, and others start in placebo and are then crossed over to drug. This way, none of the patients in the study has to be only in placebo, and it also means that each patient is their own control.
The trial measures epilepsy as the main symptom for improvement. They also track cognitive function and quality of life as additional potential areas of improvement.
What would be the next steps for approval?
This trial is only taking place at one hospital, NYU Langone Medical Center, because it is what is known as an “investigator-initiated study”. Investigator-initiated means that the company that owns ataluren was not who decided to start this trial, but the investigator (the clinician) from NYU approached the company and asked them to let them run this clinical trial at their hospital.
Because it is a small pilot trial, the resulting data will not be sufficient for requesting approval of ataluren for treating CDD. So first the drug results need to be published, it has now completed recruitment but the data collection and publication are not yet completed. And after that, if the data is positive, PTC would need to run a Phase 3 pivotal trial. So the next step to this clinical trial is another (larger) clinical trial if the results are positive.
Where can I find more information about the trial?
The clinical trial and contact information are here.
#2 GANAXOLONE – MARINUS PHARMACEUTICALS
Ganaxolone is a drug currently in Phase 3 (pivotal) trials in CDD. It is also in clinical trials for other neurological diseases. It has not yet been approved for any disease, and if everything goes well it is likely to become the first drug to be ever approved for the treatment of CDD since it is the most advanced one.
How does this drug work?
You might be familiar with medications like Valium and Xanax. They belong to a class of drugs, benzodiazepines, that are used for anxiety, insomnia and muscle relaxation among other uses. Some drugs in this class, like clobazam (Onfi) are even used for treating epilepsy. These drugs all enhance the activity of a type of brain receptors called GABA receptors and the result is “brain relaxation”. The brain of people with CDD has an excess of neuronal activity, so drugs that enhance or facilitate GABA receptor function can help reduce excessive activity and minimize some of the symptoms.
Ganaxolone binds to the GABA receptor and enhances their activity in a different way to how benzodiazepines work, so it is thought to achieve the “brain relaxation” with slightly different properties. It is also known that in some epilepsy syndromes, patients have low blood levels of a ganaxolone-like endogenous body hormone, so part of the efficacy of ganaxolone could be due to it helping correct this deficiency.
Is there any previous clinical experience with this drug?
Yes. Ganaxolone has been in clinical trials for other neurological diseases, like Fragile X syndrome and partial onset (focal) seizures in adults. More importantly, there has been a Phase 2 (pilot) trial in people with PCDH19 epilepsy and CDD that sowed it had efficacy on both patient populations when looking at their epilepsy. Because ganaxolone was also safe, it has now been progressed to the final Phase 3 (pivotal) studies in PCDH19 epilepsy and in CDD, now in two separate studies.
How is the clinical trial in CDD?
The Phase 3 clinical trial of ganaxolone in CDD is called the Marigold Study and takes place at numerous centers internationally. The trial is currently recruiting for patients. You can find more information about trial sites in the website of the Marigold study.
To enroll in the trial the patient needs to have a mutation in CDKL5, be 2-21 years old, and have at least 16 “major seizures” per month, which includes tonic-clonic seizures and atonic (drop) seizures. They are looking for at least 70 trial participants.
During the trial some of the patients are given ganaxolone while some receive placebo. Neither the families nor the physicians know which group the patient is in. After 17 weeks all patients are offered a chance to take ganaxolone, so if your child is placed in the placebo group it just means they will be starting the actual treatment about 4 months later.
The trial will measure epilepsy as the main symptom for improvement, and will also track improvement in other areas such as attention and behavior.
What would be the next steps for approval?
The Marigold study is a pivotal trial, meaning that it is a final trial. Once the study is completed, Marinus will submit all the documentation to the different regulatory agencies and request the marketing authorization for the treatment of CDD.
Where can I find more information about the trial?
Marinus has created a website specifically for this trial: the Marigold study. You can also find more information as well and some CDD materials for patients at the company website.
#3 – TAK-935 / OV935 – OVID THERAPEUTICS AND TAKEDA PHARMA
TAK-935, also known as OV935 since it is co-developed by Takeda and Ovid, is a drug currently in Phase 2 trials in CDD and other neurodevelopmental syndromes with epilepsy. It is an experimental drug and it is not yet approved for any other disease.
How does this drug work?
As you will remember from some paragraphs before, GABA is an inhibitory substance in the brain, which is why some drugs like ganaxolone enhance the activity of the GABA receptors to reduce brain excessive activity. They enhance brain inhibition. The excitatory substance in the brain is glutamate, and we know that the brains of people with CDD have an excess of neuronal activity in part due to too much glutamate signaling. What TAK-935 does is to reduce this excessive activity by reducing glutamate signaling, therefore bringing brain activity down to healthier levels. It reduces brain excitation.
Is there any previous clinical experience with this drug?
TAK-935 had already been in clinical trials for other neurological diseases although it was never taken all the way to the market. Because of that, the Phase 1 trials were already done. When Ovid and Takeda decided to test TAK-935 in drug-refractory epilepsies they run a Phase 2 (pilot) trial with 18 patients with a variety of rare epilepsy syndromes. Because the safety was good, and the patients experienced a reduction in seizures, the companies decided to progress the drug to further testing, and it is now being studied in four different Phase 2 (pilot) trials: one for Lennox-Gastaut syndrome, one for Dravet syndrome, one for Dup15q syndrome, and one for CDD.
How is the clinical trial in CDD?
The CDD and Dup15q studies are combined under a trial called the ARCADE study. The trial is currently recruiting patients, and is looking for 15 people with CDD ages 2 to 35 with at least 3 motor seizures per month.
Because it is a pilot study, there is no placebo group. All 15 participants will receive the experimental drug on top of their regular baseline medication. Participants will take TAK-935 for 20 weeks (8 weeks bringing up the dose slowly followed by 12 weeks at maintenance levels), and the total trial duration beginning to end is 30 weeks. At the end of this period all participants will be offered to keep taking the drug if they found it to be effective.
The trial will measure epilepsy as the main symptom for improvement, and will also track general improvement.
What would be the next steps for approval?
Because the clinical trial is a Phase 2 (pilot) trial, and not a final pivotal trial, the results of the trial will not be sufficient for the companies to request a marketing authorization. If the trial results are good, then they will have to run one or two Phase 3 (pivotal) clinical trials like the one currently ongoing with ganaxolone, involving many more patients and most likely a placebo group. So the next step to this clinical trial is another (larger) clinical trial if the results are positive.
Where can I find more information about the trial?
You can find more information about this trial in the ARCADE study website.
#4 - FENFLURAMINE – ZOGENIX
Fenfluramine is a drug that was approved many years ago for treating obesity as part of a combination pill. It was later discovered to have efficacy in treating drug-refractory epilepsy in an epilepsy syndrome called Dravet syndrome, and it has now completed all of the clinical trials for Dravet syndrome and is awaiting regulatory review to obtain the marketing authorization. Pilot studies have shown that fenfluramine has efficacy in other syndromes as well, so a pivotal trial is ongoing for Lennox-Gastaut syndrome and the company is interested in evaluating the efficacy of their drug in other syndromes with epilepsy to identify potential new diseases that could benefit from it. One of those, is CDD.
How does this drug work?
While GABA and glutamate are respectively the main inhibitory and excitatory substances in the brain, there are several other substances that are known as “modulatory” because they tweak brain activity in many different ways. One of these is serotonin, and you might be familiar with antidepressants increasing serotonin signaling to stabilize people mood. This is also what fenfluramine does. Fenfluramine enhances serotonin signaling through some of the serotonin receptors, of which they are in total 15 different ones each playing different roles in the brain. It is not clearly known why these serotonin receptors are involved in epilepsy, but the clinical data so far has shown that fenfluramine is a very good anti-epileptic drug, at least in children with Dravet syndrome. Fenfluramine might also bind to other receptors in the brain, this is all still being studied.
Is there any previous clinical experience with this drug?
Yes! The reason why fenfluramine will be studied in CDD is because of the results they have seen with Dravet syndrome. Dravet syndrome is another neurodevelopmental syndrome with epilepsy, and unlike CDD where some of the patients ultimately outgrow their seizures, in Dravet syndrome this doesn’t happen. Two Phase 3 (pivotal) trials with fenfluramine in these children showed that fenfluramine could reduce seizure frequency by more than 70%, and about one in four participants was seizure free or “near seizure free” (about 4 seizures a year). The side effect profile of fenfluramine is similar to other anti-epileptic drugs and it requires extra cardiac monitoring.
How is the clinical trial in CDD?
The trial is a pilot study, involving 10 patients with CDD that all receive the drug because there is no placebo group. The trial has not yet started recruiting, it will take place at NYU Langone Medical Center, and is looking for children with CDD ages 2 to 18 with more than 4 convulsive seizures a month that will receive the drug added to their baseline medication during 14 weeks.
The trial measures epilepsy as the main symptom for improvement. They also track quality of life and general improvement.
What would be the next steps for approval?
This trial is only taking place at one hospital, NYU Langone Medical Center, because it is again an “investigator-initiated study” as explained above for ataluren.
Because it is a small pilot trial, the resulting data will not be sufficient for requesting approval of fenfluramine for treating CDD. So the next step to this clinical trial is another (larger) clinical trial if the results are positive.
Where can I find more information about the trial?
You can find more clinical trial and contact information HERE, and additional information about previous results with fenfluramine in Dravet and Lennox-Gastaut syndromes HERE.
THE FUTURE - IS THERE ANY MORE RESEARCH ONGOING BEYOND THESE TRIALS?
Oh yes! These four are all therapies that were already developed for treating other diseases (most for epilepsy, ataluren for addressing one specific mutation type). This means that they could move really fast into CDD, and start clinical trials right away without needing more research or improvements on them.
But there are other very exciting programs that have been started specifically to address the genetic cause of CDD. These ones will still take a couple of years before they can start clinical trials because they have been started from scratch to be designed for CDD, and that takes time that we didn’t have to wait with the “ready for clinical trials” drugs.
As a reminder of how CDD happens, CDKL5 is both the name of a gene and the protein that it produces. Each protein in the body has a specific function. The CDKL5 protein function is to put a phosphate onto other proteins which is like an on/off switch for those other proteins. This allows CDKL5 to turn on and off many functions of the neurons. Proteins that do this are called enzymes.
We are all born with mutations that were not present in our parents, that is how evolution works, but in most cases these mutations are in non-important regions, or at least are not too damaging. When one of these mutations, however, happens in the CDKL5 gene sequence and either breaks the sequence or gives it the wrong instructions, then that person cannot produce the CDKL5 protein or produces a non-functional version. Without good CDKL5 protein, all of those functions in neurons that needed CDKL5 to put all of the on/off switches in the right configuration are now not functioning properly. This is why the deficiency in CDKL5 is so bad for the brain.
There are two main efforts to fix these problems in patients with CDD. Not to treat their symptoms, but to correct the faulty biology that is causing the symptoms. These are the type of approaches that in the patient community we often call cures, although you will not hear this word from pharmaceutical companies. They prefer to call them disease-modifyingtreatments because they change the disease.
The first approach is gene therapy. If you could give each neuron a new copy of the CDKL5 gene then they will be able to produce the protein and function as a normal cell. There are multiple efforts going on in this area, but I will highlight the program from the company Ultragenyx, who announced last October that they will develop a virus carrying the CDKL5 gene as a gene therapy for CDD.
The second approach is to simply add to the brain the CDKL5 protein. This has been done in the past in other diseases caused by enzyme deficiencies, and are known as Enzyme Replacement Therapies. I would highlight here that the company Amicus has been working on this approach for the last couple of years, trying different approaches to deliver the CDKl5 protein to the brain.
This means that in the next couple of years we will have several clinical trials for CDD that target disease symptoms, and then we will start having clinical trials with therapies that target the cause of the disease. The first group of drugs will reduce the symptoms of the disease and give the patients a chance to acquire more skills faster while they have less seizure burden. The second group of therapies, in particular when used in very young kids, will lead us to a future where children born with mutations in CDKL5 will pretty much be able to grow as if their gene was not mutated. As usual in medicine some trials will fail, but other trials will get started. The important message is that there are so many programs ongoing that the question is not IF we are going to make it to effective therapies, but WHEN.
Let me know if you have some questions on these trials that I didn’t cover in the article!
Ana Mingorance, PhD
Main Lessons from the World Orphan Drug Congress USA 2019
Many orphan drugs are advanced therapies. Pricing and access are major issues. Epilepsy is catching up with gene therapy. We shouldn’t call them rare diseases, but frequently misdiagnosed diseases. Either we wait 2,000 years for treatments or we start thinking “many diseases at a time”, and online patient communities are now part of the drug development process. That’s the short summary of the main lessons I took home from attending the World Orphan Drug Congress at the National Harbor April 10-12. The WODC one of the largest meetings dedicated to the development of new medicines for rare diseases and takes place once in the US and once in Europe every year. In a bit more detail, here is the expanded list of what I would like to share with you from the conference.
Many orphan drugs are advanced therapies. Pricing and access are major issues. Epilepsy is catching up with gene therapy. We shouldn’t call them rare diseases, but frequently misdiagnosed diseases. Either we wait 2,000 years for treatments or we start thinking “many diseases at a time”, and online patient communities are now part of the drug development process.
That’s the short summary of the main lessons I took home from attending the World Orphan Drug Congress at the National Harbor April 10-12. The WODC one of the largest meetings dedicated to the development of new medicines for rare diseases and takes place once in the US and once in Europe every year.
In a bit more detail, here is the expanded list of what I would like to share with you from the conference:
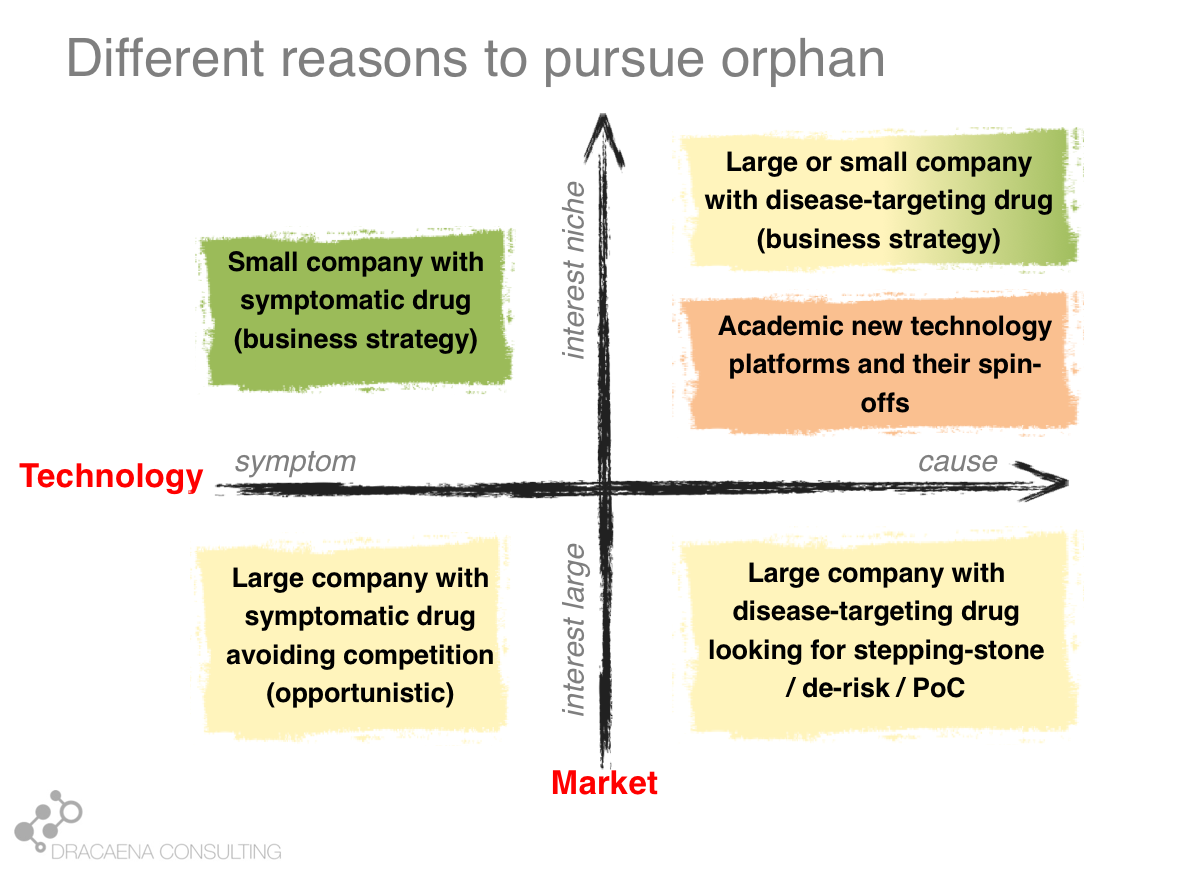
1- A lot of “orphan drugs” use new technologies.
Although the Orphan Drug Act was conceived to stimulate development of therapies for rare diseases in general, and it has often been used in an “opportunistic way” for drugs that could have targeted broader populations, the new technologies that allow us to target specific gene defects are taking over the field. There was an entire day on Next Generation Therapies that highlighted programs in development using antisense, base editing, gene therapy and cell therapy approaches. There was also a great plenary session with multiple of these approaches including companies already in the clinic such as Alnylam, Sangamo Therapeutics and CRISPR Therapeutics.
I believe that with most rare diseases having a genetic cause, and most genetic diseases being rare diseases, it is only natural that gene-targeting technologies will find their home in the rare disease space.
2- Big challenges: delivery, manufacturing and drug pricing
In many of the debates the conversation went beyond the medical use of these new technologies and focused on some important challenges that the orphan drug community needs to face and address. One important issue that multiple of the new technologies face is how to get into their target tissue. This is particular complex for neurological diseases, although some companies are making great progresses in this space and Alnylam announced a few days before the WODC a partnership with Regeneron to apply their RNAi therapeutics to ocular and neurological diseases. Manufacturing challenges also limit cell and gene therapy development, making many of these one-time treatments too expensive. And that was indeed one of the main topics of discussion in the meeting: pricing and affordability of these new therapies. Emil Kakkis from Ultragenyx stressed the importance of ensuring access to therapies saying that this is the golden age for rare diseases and yet a therapy means nothing if patients cannot get access to it. While delivery and manufacturing are technical challenges, identifying suitable payment mechanisms that guarantee patients access to treatments is a social challenge that is likely to be the hardest to address.
3 – Epilepsy meets the future
I regularly participate in orphan drug conferences, where I get to see all these exciting new technologies, yet at the epilepsy conferences (my field) most of what we see is traditional pharmacology that focuses on reducing brain activity without targeting the specific cause of the epilepsy in that patient. I work on neurological syndromes with epilepsy, and a very large number are monogenetic meaning that they would be the ideal target for these new approaches. Barry Ticho from Stoke Therapeutics said it very clearly at the WODC:
“there are more than 100 epilepsy genes but not a single therapy that treats the causes”.
But that is changing.
At the WODC we could see multiple approaches in development to specifically target the causes of several neurodevelopmental syndromes with epilepsy. In addition to Stoke presenting their antisense approach for Dravet syndrome, the small company RogCon presented their antisense approach for SCN2A epilepsies, Roche presented their antisense approach for Angelman syndrome, and as part of a panel, Xenon Pharma highlighted their small molecule approach with a potassium channel opener for KCNQ2 epilepsy. This is a good reminder that for some genetic diseases, small molecules could also offer an excellent disease-targeting approach, as also seen in cystic fibrosis where different CFTR protein alterations are treated by specific molecules designed to address those alterations.
To prepare the field of epilepsy for these new personalized approaches, early genetic diagnostic is paramount. Invitae has now extended the Beyond the Seizure epilepsy program to offer free genetic testing to all US children under 60 months of age with epilepsy. This program is sponsored by BioMarin, Stoke and Xenon, and I hope it will get more sponsorship to be opened to patients currently older than 5 years old and from other countries. Having a genetic diagnosis (early or not) is still one of the big challenges for rare disease patients around the world.
4- Don’t call them rare – the importance of the words we use
Carol-Anne Partridgeis the Founder of a patient charity, and the mother of a beautiful girl with CDKL5 Deficiency Disorder. In her presentations, Carol-Anne always stresses that we need to change the narrative around rare diseases. Her experience resonates with that of many other people with rare diseases and their families, tired of a focus on what they cannot do (instead of what they can do, or the future cures they will have), and physicians talking in front of their kids in a way that doesn’t respect them. We need to change the narrative to one of hope and positivity and respect for people with rare diseases.
Arndt Rolfs, CEO of Centogene, made a similar plead to the WODC audience about the need to change the narrative. If we call them rare diseases, he explained, physicians will think that they will never across one, so they will not watch out for rare diseases. We should call them frequently misdiagnosed diseases instead, so that physicians are particularly alert to not miss them. This makes so much sense, and has such important consequences to patient diagnosis, that we should all start using the “frequently misdiagnosed diseases” term a lot more often.
With his presentation, Dr Rolds echoes Carol-Anne’s message: we need to change the narrative around rare diseases if we want to diagnose, treat and respect people with rare diseases.
5 – Reshaping “Rare” by thinking “many diseases at a time”
Chris Austin, Director of NCATS, gave one of my favorite presentations at the WODC about reshaping “rare”. At the current rate of orphan drug approval it will take 2,000 years before we have treatments for all rare diseases (assuming each new drug is approved for a different rare disease, which is not the case). A radical change in the way we discover and develop these therapies is needed. Chris’ proposal is to move from working on “one disease at a time” to working on “many diseases at a time”, exploiting commonalities among rare diseases and platform technologies to diagnose them and treat them. NCATS has multiple initiatives around this “many diseases at a time” approach, from empowering patient communities to get active in research to providing guidance on interoperable registries and partnering in drug development, including developing a gene therapy platform.
While Chris advocated for these multiplexing approaches to diagnosis and therapy development, I think that we will need some regulatory innovation to address the challenge that in some fields companies are forced to run pivotal trials in a rare disease at a time, making it not viable for companies to run trials in ultra-rare diseases and condemning these patients to off-label drug use (more thoughts on this regulatory challenge here).
6 – The place for social media and on-line patient platforms in orphan drug development
An interesting topic that also was present in different tracks at the WODC was the importance of social media and digital platforms in research and development. Luke Rosen, Head of Patient Engagement at Ovid Therapeutics, explained how Ovid believes that listening to the patient community voice is crucial for companies to understand what truly matters to families affected by rare diseases, and how Ovid is supporting the conversation by helping create community sites. A patient community that comes together becomes a stronger community, and an essential part of the drug development process. And Luke understands this well because he is also the Founder of the KIF1A.org Foundation to develop therapies for his daughter and other children with KIF1A associated neurological disorder.
We also had a workshop during the first day of the WODC, where I participated, where we reviewed how on-line communities are able to capture the patient voice in a way that is directly usable for drug development.
DuchenneXchange, for example, was created to help connect, educate and inform the Duchenne muscular dystrophy community, and James Valentine explained how regulators appreciate the use of on-line platforms to collect patient experience and preference data that will be useful for regulatory purposes. In fact in the FDA guidelines for Patient Focused Drug Development meetings, one of the methodologies for collecting patient experience data are precisely “Social Media and Identifiable Patient Communities”.
And “community” is perhaps the main value of the World Orphan Drug Congress. As much as I enjoy the workshops and presentations and roundtables, the main value I get from attending the WODC is the network. I get to catch up face to face with people I already knew, and I get to meet new people from promising companies or related patient communities.
See you all next year!
Ana Mingorance PhD
Dravet syndrome gene therapy
There are multiple gene therapy programs in development for Dravet syndrome including those that supply and extra copy of the SCN1A gene and those that boost expression from the healthy SCN1A gene copy. Clinical trials are around the corner, with Stoke Therapeutics expecting to initiate clinical trials in 2020. Just Stoke is not enough. New corporate players, and ideally some precompetitive collaboration around the common challenges of validating clinical outcome measures and biomarkers, are needed to maximize the success of gene therapies for Dravet syndrome.
See gene therapy update in September 2020
One of the top google searches that brings people to my website is “Dravet syndrome gene therapy”.
I often review the Dravet syndrome pipeline (recently HERE and HERE, notably HERE), but so far we haven’t had yet any clinical trials with gene therapy in Dravet syndrome so those treatments are largely not in the reviews. Nevertheless, it is understandable that gene therapy is the most attractive therapy for people with Dravet syndrome.
Here is a review of the gene therapies in development for treating Dravet syndrome, how each of them works, and when they are expected to start clinical trials.
CURRENT GENE THERAPIES IN DEVELOPMENT FOR DRAVEY SYNDROME
In diseases like Dravet syndrome where the problem is that a copy of the gene is missing or not functional due to mutations, the desired therapy is one that can restore normal gene expression and therefore normal protein production. In other words, we need more protein.
In the case of Dravet syndrome, the gene is SCN1A, and the protein that is needed is the neuronal sodium channel Nav1.1. As a result of mutations in the gene, the number of Nav1.1 channels at the neuronal surface is not sufficient, there is less sodium crossing the membrane, and the neuron cannot fire properly. The result is Dravet syndrome.
One particularity of Dravet syndrome is that only one of the two copies of the SCN1A gene is affected, the second one is perfectly fine, so that second copy can serve as the supply for extra protein production. As you will see, the most advanced programs are exploiting this possibility.
Broadly speaking, there are two approaches to restore protein expression in Dravet syndrome: you either supply the cell with an extra healthy copy the gene, which will lead to more protein being produced, or you try to boost the expression of the healthy gene.
(1) Supply a new copy of SCN1A
When people think about “gene therapy”, the type of therapy they are thinking about is the one where the DNA of a virus gets replaced by the gene that the person needs, and that modified virus is used as a Trojan horse to infect cells and deliver them the therapeutic gene.
If the SCN1A wasn’t so large that it cannot fit the most commonly used virus for gene therapy, the Adeno-Associated Virus (AAV), we would probably have clinical trials right now using AAV-based gene therapy for Dravet syndrome. A year ago I reviewed this problem in the article “big gene, small virus”.
However the large size of the gene has so far kept all gene therapy companies away from working with viral vectors for Dravet syndrome, and only academic groups are trying to push the current comfort zone of AAV gene therapy into a new era where we can use virus to deliver large genes. The good news is that there are multiple labs working on this, so there are multiple shots on goal:
|| Dravet Canada and the US Dravet Syndrome Foundation are supporting a gene therapy project at Toronto University that is developing a gene therapy for Dravet syndrome although not details are available on the approach used (virus or gene).
|| The Spain-France-Israel consortium CureDravet started a year ago to develop a gene therapy for Dravet syndrome using Adenovirus, a type of high-capacity virus that is large enough to contain the entire SCN1A gene (Strategy 1 in the figure). They are collaborating with Dravet Syndrome Foundation Spain and the Dravet Syndrome European Federation, and have also developed a close relationship with patient groups.
|| At UCL, the team of Rajvinder Karda is working on two approaches. One is to use another type of large-capacity virus, Lentivirus, to carry the SCN1A gene (Strategy 1 in the figure). The second approach uses two AAV virus, each containing half of the SCN1A gene, which are able to recreate the full channel once they co-infect the same cells (Strategy 3 in the figure). They have received the support from Dravet Syndrome UK and share updates with other interested patient groups.
All of these projects are in early preclinical stages, and they have not yet published a proof of concept in a Dravet syndromemouse model, which is an initial stage prior to advancing the treatments towards clinical trials. These programs are therefore all years away from clinical trial initiation, with no guarantee of succeeding.
(2) Boost expression of SCN1A
Another strategy that has been used successfully in other diseases is to use small fragments of RNA (oligonucleotides) to boost the expression of a gene either without needing to add an external gene copy with a virus. This one is the strategy most advanced for Dravet syndrome.
|| The first program to be developed was OPK88001(previously CUR-1916) by OPKO Health. The therapy is a piece of oligonucleotide that binds to the DNA and removes an endogenous repressor of SCN1A (Strategy 3 in the figure). As a result, the good copy of SCN1A experiences much more transcription, leading to more mRNA and more Nav1.1 protein levels. The company expected to initiate clinical trials as early as in 2017, later announced to be in 2019, and as of February 2019 there are no news of when the program will be able to move into the clinic.
|| 2018 brought the good news that Stoke Therapeutics was developing an antisense oligonucleotide treatment to boost expression of SCN1A as well. This oligonucleotide binds to a form of mRNA and leads to an increase in the levels of mature mRNA and Nav1.1 protein (Strategy 4 in the figure). The company has shared preliminary data with efficacy in a mouse model and is planning to initiate clinical trials in 2020.
|| Last, an academic group in Italy, with funding from CURE and the Dravet Syndrome European Federation, is researching an alternative approach to boost production of Nav1.1 protein from the existing mRNA through another oligonucleotide approach (Strategy 4 in the figure).
REMAINING NEEDS FOR GENE THERAPY IN DRAVEY SYNDROME
(1) Questions to answer
Is overexpression of SCN1A bad? This is one important question that the field needs to answer so that we know if an excess of sodium channel as a result of the gene therapy could have negative consequences. This has been seen in some diseases where there are patients with duplication of the gene, for example in Rett syndrome where patients with Rett syndrome have a bad copy of MECP2 but there is also another disease caused by duplication of MECP2. In that case, increasing MECP2 levels too much with gene therapy would convert neurons from MECP2-deficient (Rett syndrome) into MECP2 duplication!
There appears to be no negative consequences of mild overexpression of SCN1A but there has been no clear study how much increase is enough and how much is too much. This is one science gap important for gene therapy.
Other questions impact how to design a clinical trial with a gene therapy in Dravet syndrome. So far clinical trials measuring seizure frequency have been very successful, but a gene therapy is expected to improve the syndrome beyond just seizure frequency. Unfortunately, the field of Dravet syndrome is still immature when it comes to clinical outcome measure development and validation for non-seizure outcomes (for non-scientists in the audience: we don’t know how to quantify improvements of the disease in a clinical trial beyond seizures).
Also, all of these approaches are increasing the levels of Nav1.1, yet we don’t have any biomarker that could help us see what are the levels of functional or total Nav1.1 in patients. Imagine a clinical trial where a dose of the treatment is ineffective. If we don’t know if the dose had succeeded at restoring Nav1.1 levels, how would we interpret that trial?
(2) New players needed
While it is exciting to see so many academic groups testing new forms of gene therapy for Dravet syndrome, I would like to see more companies in this space, in particular in the viral-mediated therapies. Beyond achieving the initial mouse proof of concept, the development of these therapies will face important challenges such as safety testing, scale up and manufacturing, clinical trial design able to measure non-seizure outcomes and biomarkers, and the massive cost of clinical trials. These challenges require the involvement of companies with the expertise and funding that can take these discoveries into the clinic and into the market, so the involvement of more companies in the gene therapy space for Dravet syndrome will be a necessary step as the pipeline progresses.
With Stoke now leading the development of oligonucleotide therapies for Dravet syndrome, it feels safe to think that the probabilities that we will have the first disease-modifying clinical trial for Dravet syndrome in 2020 is very high, and that an antisense therapy will reach the clinical trial stage. It is much less clear whether any of the current viral strategies will reach clinical trials since there is no corporate involvement. Antisense therapies and therapies with viral vectors have different advantages and disadvantages in the clinic. Because of that, I hope to see the antisense oligonucleotide approach from Stoke followed into the clinic by some company with a viral-mediated gene therapy approach, as it has happened in other fields such as SMA.
IN SUMMARY
There are multiple gene therapy programs in development for Dravet syndrome including those that supply and extra copy of the SCN1A gene and those that boost expression from the healthy SCN1A gene copy.
Clinical trials are around the corner, with Stoke Therapeutics expecting to initiate clinical trials in 2020.
Just Stoke is not enough. New corporate players, and ideally some precompetitive collaboration around the common challenges of validating clinical outcome measures and biomarkers, are needed to maximize the success of gene therapies for Dravet syndrome.
Do you know of any other gene therapy project that I missed? Let me know in the comments.
Ana Mingorance PhD
Expected Dravet syndrome news during 2019
2019 will be the year when we might have the European launch of Epidiolex, the US approval and launch of Fintepla, an ongoing clinical trial with TAK-935, hopefully some news about the ability of Translarna to improve Dravet syndrome by rescuing some of the nonsense mutations, and a year to prepare for the clinical trials that starting in 2020 will dominate the field: gene therapy approaches for Dravet syndrome that will treat more than just seizures. This entry reviews when we expect the main news about the Dravet syndrome pipeline during 2019.
2018 saw many progresses in the Dravet syndrome drug pipeline, including major milestones such as the approval and launch of Epidiolex in the US, the completion of a second very successful pivotal trial with fenfluramine, the initiation of clinical trials in Dravet syndrome by Ovid and Takeda and the appearance out of the blue of Stoke Therapeutics, with an antisense approach for restoring Nav1.1 expression in Dravet syndrome.
Some of these news were already anticipated by the companies at the beginning of the year (reviewed here), so we knew when to expect them. But other took us by surprise, mainly around the programs that were less advanced at the beginning of the year.
Keeping that in mind, here is what we can expect in 2019 from the Dravet syndrome programs from GW Pharmaceuticals, Zogenix, Ovid Therapeutics and Takeda, PTC Pharma and NYU, OPKO Health, and Stoke Therapeutics, based on what these companies have communicated.
Epidiolex (cannabidiol) – GW Pharmaceuticals
The decision from the EMA to approve or not Epidiolex for the treatment of Dravet and Lennox-Gastaut syndrome is due within the first quarter of 2019, and if the decision is positive, GW hopes to start the first national launches of Epidiolex starting as early as Q2 2019.
Fintepla (fenfluramine) – Zogenix
Zogenix also expected to deliver good news during the first quarter of 2019, when it planned to complete the submissions of the marketing authorizations for the two largest markets (called NDA in the US and MAA in Europe). This was one of the first news that we had in 2019, since the successful double-submission was announced at the beginning of February.
We should have the news about the FDA decision on Fintepla in Q3 2019, followed by a launch in the US market before the end of the year. For the approval and launch in Europe we will have to wait until 2020.
Translarna (ataluren) – PTC Therapeutics / NYU
Last year we anticipated to get the results of the Phase 2 clinical trial with ataluren in children with Dravet syndrome caused by nonsense mutations during the second quarter of 2018. However the clinical trial has not yet been completed (according to clinicaltrials.gov it is active but not recruiting and is still ongoing in the last PTC pipeline review). In the absence of any public estimates on trial completion, all we can estimate is that if the trial has completed enrollment, and based on the trial protocol duration, we might expect to hear news by the end of Q2 2019.
TAK-935 (OV935) – Ovid Therapeutics / Takeda
Even before completing the Phase2a basket trial in adult patients with different developmental and epileptic encephalopathies, Ovid and Takeda announced the initiation of a Phase 2 clinical trial in pediatric patients with Dravet syndrome and Lennox-Gastaut syndrome. The trial, called ELEKTRA, is currently recruiting and based on the company last estimates it will continue to enroll during 2019. This means that we might or might not get the news of the next milestone for this program for Dravet syndrome during 2019, which would be the completion of trial enrollment.
OPK88001 – OPKO Health
Over the years OPKO has provided very limited information on their program targeting Dravet syndrome with an antisense therapy. 2017 materials had indicated that the therapy, OPK88001, would be ready to start clinical trials in late 2017, which was later to moved to be planned to start somewhere during the first half of 2018. The latest corporate update, from June of 2018, still lists the program as active and indicates the Phase 2 trial will start during the second half of 2018. There were no news about the trial initiation and there are no more news about this program. The program is still listed as active in the last company presentation of September 2018 but no timelines were provided. We are therefore not able to predict if we will hear any news from this program during 2019.
Antisense Oligonucleotide - Stoke Therapeutics
One of the big news of 2018 was Stoke Therapeuticscoming out of stealth mode with an antisense oligonucleotide approach to restoring expression of the protein missing in Dravet syndrome, and plans to bring the antisense therapy into the clinic by 2020. Although the company has not communicated any expected release of news to take place during 2019, it is predictable that during 2019 Stoke will announce the clinical trial plans, and communicate/publish more complete preclinical proof-of-concept data that supports their clinical trial plans.
Other surprises
During the American Epilepsy Society meeting in December of 2018, the company Encoded Genomics, still in stealth mode, appeared as a new company developing a gene therapy approach for the treatment of Dravet syndrome. The company sponsored the Dravet Syndrome Roundtable and were open about the fact that they are developing such therapeutic approach, although no more details were given. If Encoded or any other new company confirms that they are working on a gene therapy for Dravet syndrome, and releases some news during 2019, it will solidify the transition of Dravet syndrome from a disease that we manage with symptomatic anticonvulsant medications to a disease that we can start targeting with a variety of gene therapy approaches.
SUMMARY
2019 will be the year when we might have the European launch of Epidiolex, the US approval and launch of Fintepla, an ongoing clinical trial with TAK-935, hopefully some news about the ability of Translarna to improve Dravet syndrome by rescuing some of the nonsense mutations, and a year to prepare for the clinical trials that starting in 2020 will dominate the field: gene therapy approaches for Dravet syndrome that will treat more than just seizures.
Ana Mingorance, PhD
Sources:
[1] GW Pharma company presentation January 2019
[2] Zogenix investor update December 2018 and press release 6 February 2019
[3] ClinicalTrials.gov information for NCT02758626
[4] Ovid Therapeutics Press Release 4 January 2019
[5] OPKO Health company presentation September 2018
[6] Stoke Therapeutics press release December 2018
Announcements within the same quarter ordered by drug name (alphabetic).
Orphan drug approvals: 2018 set new record for the EMA, but some red flags
2018 saw a record year in orphan drug approvals in Europe, but there are reasons to worry. Year after year, the number of orphan drug approvals in Europe is only one fifth to one third of the number of drug approvals in the US. Also, if orphan designations represent an early marker of the orphan drug development trend, then we might expect a decrease in the number of approvals in the immediate future. This article reviews the number of orphan drug designations and approvals in Europe in the 2000-2018 period to understand the trends that might impact the number of orphan drug approvals in the next few years.
In 2018, the European Medicine Agency (EMA) issued 84 positive opinions on new medicines. While the total number is lower than the year before, when 94 programs received approvals, the number of drugs being approved for the first time in Europe was higher than in 2017 (42 versus 35), meaning that there was more innovation reaching the market.
In this month of February, when we celebrate and promote awareness on rare diseases, I would like to review how 2018 looked when it comes to orphan drug approvals, and orphan drug designations (data from the EMA).
The first observation that stands out when looking at the graph of orphan drug designations in Europe is a notable drop in the last two years when compared to the previous trend (Aqua).
The second observation is the progressive increase in orphan drug approvals (yellow), although quite far from the number of orphan drug designations being issued.
Let’s discuss those two trends and what they might mean about the future of orphan drugs in more detail.
2018 Orphan Drug Designations
While we often refer to them as orphan drugdesignations and orphan drugapprovals, these are the notations used by the FDA. In fact, the EMA and the European Commission (which is the ultimate organism authorizing a drug approval) prefer to talk about “orphan medicinal products” instead.
The number of orphan medicinal product designations has grown much since the process was started in 2000, and reached a peak during the years 2014-2016 with designation numbers of around 200 per years. While the drop in 2017 to numbers just shy of 150 could have been a fluke, 2018 has confirmed the decrease in the number of drugs obtaining the orphan status, indicating there might be some difference in the trend.
One possibility is that the decrease in orphan designations is due to a decrease in the number of applications. However the number of designations (successful applications) in 2017-2018 is comparable to 2012-2013, yet the number of applications was 25% higher in 2017-2018 meaning that the success rate was lower.
A breakdown of these numbers indicates indeed that the success rate as decreased, and in the recent years the percentage of successful orphan applications has gone down from about 70-76% to a recent low of 59% in 2017. However 2018 has returned to the rate of previous year with 67% of the applications receiving a favorable opinion. Therefore, a reduced success rate due to the EMA becoming stricter or applications becoming weaker cannot fully explain why for the last two years we are seeing a substantial decrease in the number of orphan medicinal product designations in Europe.
2018 Orphan Drug Approvals
The number of orphan drug approvals in Europe continues to experience a progressive increase over the years. In the last 5 years, for example, we had an average of 18 approvals of orphan drugs year, up from 7-8 during the previous ten years.
The actual number of orphan medicinal products is a bit smaller. For example, in 2018, a total of 21 orphan medicinal products were approved for a total of 26 orphan therapeutic indications, meaning that some drugs had approvals for multiple rare diseases.
Both of these numbers, 21 orphan medicinal products approved for 26 orphan therapeutic indications, are a record, exceeding any previous year and making 2018 the best year for orphan drug approvals in Europe.
The upward trend in the number of new orphan drug approvals does not reflect the regression in the number of designations experienced during 2017 and 2018. This is possibly due to the fact that most products receive the orphan drug designation years before they get approved, and raises the question of whether we could expect to see a decrease in the number of orphan drug approvals as soon as the smaller generation of “2017 and 2018 designations” reaches the finish line.
Therefore, although 2018 was a record year in orphan drug approvals in Europe, it is expectable that we will see some reduction in the next few years.
2018 – US FDA vs EMA
2018 was the best year for orphan drugs in Europe, with 21 different drugs obtaining marketing authorization for 26 orphan indications. Yet when we compared these numbers with the orphan marketing authorizations issued by the FDA during the same time period the European numbers are dwarfed. Year after year, the number of orphan drug approvals in Europe is only one fifth to one third of the number of drug approvals in the US.
It is also clear form the graph that the lengthier approval process in Europe is not responsible for these lower numbers, since the dips and peaks of both graphs are identical and do not suggest a simple delay in Europe.
As an advocate for the rare disease patient community in Europe, these numbers worry me and trigger more questions than they address. For example, what does this mean for patients with rare diseases in Europe? Are they accessing the US-approved drugs through some medication import mechanisms or are we looking at a massive drug access problem in Europe? We need to also take into consideration that these graphs represent only central approvals, and that each country in the EU has to give the manufacturer green light to launch in that country, which due to price negotiations or poor market outlook is limited to only a fraction of the countries in the EU.
In summary:
2018 was a record year of orphan drug approvals in Europe
The trend matches the US trend, also with a record of approvals in 2018
However the number of orphan drugs approved in Europe is much smaller than in the US, meaning less options for patients
The number of orphan drug designations (before approval) in Europe has fallen in the last two years
If orphan designations represent an early marker of the orphan drug development trend, then we might expect a decrease in the number of approvals in the immediate future
Ana Mingorance, PhD
2018 in review: Dravet syndrome milestones
With 2018 now behind us, it is time to review how well companies working on Dravet syndrome delivered based on the timelines that they had announced at the beginning of the year. While it is hard to predict exactly when many milestones are going to happen, in particular those still over half a year away or more, the class of 2018 did quite well overall, and many of the news that we were expecting took place on schedule – with some exceptions.
With 2018 now behind us, it is time to review how well companies working on Dravet syndrome delivered based on the timelines that they had announced at the beginning of the year. This was the summary figure that I published a year ago outlining what to expect in 2018:
So how did it go?
While it is hard to predict exactly when many milestones are going to happen, in particular those still over half a year away or more, the class of 2018 did quite well overall, and many of the news that we were expecting took place on schedule – with some exceptions.
Ataluren – Phase 2 clinical trial
Ataluren (Translarna, by PTC Therapeutics) is being tested in a double-blind placebo controlled Phase 2 trial in patients with Dravet syndrome and CDKL5 Deficiency Disorder due to non-sense mutations. Although early last year the trial was expected to have results by Q2 of 2018, the trial is not yet completed and so the results have not been communicated. This is an investigator-indigitated trial involving only one clinical site so any delays in recruitment in that site cannot be buffered by recruitment elsewhere and has therefore a large impact on the trial timelines.
Epidiolex – FDA approval and US launch
2018 promised to be a very exciting year for GW Pharma (Greenwich Pharmaceuticals in the US) and it did not disappoint. Every expected milestone came successfully and on time.
The first milestone was the FDA marketing authorization that came as planned in Q2 2018, following a very successful FDA advisory committee meeting some months before. After approval, the DEA rescheduled Epidiolex (cannabidiol) in Q3, enabling a successful market launch in Q4 (November). Epidiolex is currently available in all 50 states.
GW also expected the announcement of the results of the second pivotal trial in Dravet syndrome to take place during the second half of 2018. The results were announced in November and matched the previous three positive pivotal trials in Dravet syndrome (one) and Lennox-Gastaut syndrome (two).
All in all, a very good year for GW, that expects the European marketing authorization in the first quarter of 2019.
Fintepla – Second pivotal trial data and regulatory filing
Zogenix also had a very good 2018, with the expected timelines being only slightly optimistic. The announcement of the results of the second pivotal trial with Fintepla (fenfluramine) in Dravet syndrome planned for Q2 ended up coming in July, and confirmed the first pivotal trial results showing unprecedented efficacy in this very difficult patient population.
The NDA submission was initiated on schedule in Q4, but is expected to be completed during Q1 of 2019, together with the European MAA, after Zogenix communicated that after pre-NDA discussions with the FDA they had decided to “conduct some additional analyses of our clinical data that could positively impact our product label” which would delay the submission of the final sections of the NDA by just a couple of months.
So, all in all, also a very good year for Zogenix.
OPK88001 – Initiation of first clinical trial
Over the years OPKO has provided very limited information on their program targeting Dravet syndrome with an antisense therapy. 2017 materials had indicated that the therapy, OPK88001, would be ready to start clinical trials in late 2017, which was later to moved to be planned to start somewhere during the first half of 2018. The latest corporate update, from June of 2018, still lists the program as active and indicates the Phase 2 trial will start during the second half of 2018. There are no further news about this program.
But just as the patient community wondered if the therapy from OPKO will ever move into the clinic, Stoke Therapeuticscame out of stealth mode with another antisense oligonucleotide approach to restoring expression of the protein missing in Dravet syndrome. So while the program from OPKO might not have reached the milestones that it expected to reach this year, a strong contender has appeared and has plans to bring the antisense therapy into the clinic by 2020.
OV935 / TAK-935– Results from the basket trial
The last clinical trial news that were expected for 2018 were the results of the Phase1b/2a basket trial with patients with mixed epilepsy syndromes that Ovid Therapeutics and Takeda were running with their molecule. The companies had planned to release the top data of this trial in the second half of the year, and just as we were wrapping up the year the trial results were announced, meeting another successful milestone. The trial had very promising efficacy data in a group of patients that included cases of Dravet syndrome, Lennox-Gastaut syndrome and other rare epilepsies.
Even before the completion of the pilot trial, Ovid and Takeda decided to move forward with additional clinical trials with OV935 (TAK-935) in four rare epilepsies, announcing in September of 2018 the initiation of a placebo-controlled Phase 2 clinical trial in Dravet syndrome and Lennox-Gastaut syndrome, and a smaller open-label Phase 2 trial in CDKL5 Deficiency Disorder and Dup15q syndrome.
So for Ovid and Takeda, 2018 delivered even more for Dravet syndrome 8and for OV935) than they had envisioned at the beginning of the year.
In the next article I will review the news that we can expect in 2019 from those programs that are in clinical trials for Dravet syndrome.
Ana Mingorance PhD
Engaged for 20 years: an orphan drug designation from 1995 just got approved in 2018
The Food and Drug Administration (FDA) set a new record in 2018 with the highest number of new drug approvals in the last two decades. The FDA also set a new record in orphan drug approvals in 2018, granting 86 new marketing authorizations for drugs treating rare diseases. In this article I review the delay between orphan drug designation and orphan drug approval, and identify how in many cases orphan drugs wait 10 or more years after reviewing the orphan drug designation and before they get approved.
The Food and Drug Administration (FDA) set a new record in 2018 with the highest number of new drug approvals in the last two decades.
With 59 new molecular entities approved, 2018 represents a big departure from a disappointing 2016 when the FDA approved only 22 new drugs, the lowest number since 2010.
The FDA also set a new record in orphan drug approvals in 2018, granting 86 new marketing authorizations for drugs treating rare diseases.
This number is higher than the total number of “new drug approvals” because not all of these drugs are new to science. Some of the drugs being approved for a rare disease had been previously approved for treating other diseases, so they count as an orphan approval but not as a “new drug” approval. Other drugs, such as Epidiolex (cannabidiol), are indeed new drugs, but because they are being approved to treat two orphan indications, in this case Dravet syndrome and Lennox-Gastaut syndrome, they count as two in the list of orphan drug approvals.
BEFORE APPROVAL: ORPHAN DRUG DESIGNATIONS
An interesting observation is that despite having more approvals of orphan drugs in 2018 than in prior years, the number of orphan drug designations for 2018 was much lower than in the previous year, with 335 designations in 2018 versus 477 a year before.
This happens because orphan drug designations are granted to drugs that intent to treat a rare disease while they are still at some point during development, prior to marketing authorisation. Some drug sponsors might request and obtain the orphan drug status (designation) many years before their drug is approved, potentially at a preclinical stage as soon as they have compelling data in animal models to support a possible benefit for patients with that rare disease. This happens in approximately one third of the orphan drug designations that are granted. In other cases, the drug sponsor might seek the designation after obtaining clinical data, usually in a Phase 2 clinical study, so for that particular drug the time between obtaining the orphan drug designation and the marketing authorisation might be much shorter.
Essentially, the orphan drug designation is like an engagement ring that the regulators grant to a drug in development for rare diseases.
And just like with engagements, it is expected that some time after that ring will come a marriage, although this is not always the case. Indeed, not all drugs that obtain an orphan drug designation during their development end up successfully reaching the market, and only a fraction of all orphan designated drugs become actual approved drugs that reach the patients.
This is very important, because within the patient community there is the expectation that most if not all of the drugs that obtain an orphan drug designation for their disease will eventually reach them. And more importantly, it is tempting, and common, to believe that once a drug obtains the orphan designation, it will not take much longer before it gets approved.
But what does “not much longer” mean in this case?
I have used the data that the FDA has released about all of their orphan drug approvals for 2018 to analyse this very important question:the delay between orphan drug designation and orphan drug approval.
THE 20 YEAR ENGAGEMENT BETWEEN ORPHAN DRUG DESIGNATION AND ORPHAN DRUG APPROVAL
The FDA granted 86 marketing authorizations in 2018 for drugs treating rare diseases. The FDA also makes the date of the orphan drug designation for each of these drugs available, so it is possible to track the time that it took them to get from designated to approved – and the numbers are not pretty.
What you see in the figure is all of the 86 orphan drug marketing authorisations from 2018 ranked by the number of years that it took them to get from designation to eventual approval in 2018. Again: those are years, not months.
In sixteen of these approvals, the drug had been designated as an orphan drug for treating that rare disease 10 or more years before it was eventually approved. In two cases it took 20 or more years.
Many of the approvals were for drugs that had received the orphan drug designation4 to 8 years before marketing authorisation.
The distribution is so broad that it means we cannot use the number of orphan drug designations for a disease, or the date of the designations, as an estimate of when that disease will see a drug approved. It might end up with 3 drugs approved in 3 years, or waiting 20 years to get the first drug approved. Every drug approved in a single year has a very different story of how it got there, and how look it took it.
THE STORIES BEHIND THOSE DIFFERENCES
To understand a bit better what leads to such a large difference in times from orphan drug designation to drug approval, we can look at the story behind the 5 approvals for drugs treating epilepsy syndromes. These are the ones highlighted in yellow in the graph.
The two drugs that tool more than 9 years to progress from orphan drug designation to approval are everolimus and stiripentol.
I have written about everolimus before. Everolimus (Afinitor, by Novartis) is similar to rapamycin, and had been already approved for multiple indications in transplantation medicine as well as for treating Tuberous Sclerosis Complex (TSC), a rare genetic disease. Everolimus obtained the orphan drug designation for treating TSC in 2009, and was first approved under that designation for treating a type of tumor characteristic of TSC in 2010. After seeing that the treatment also had efficacy in treating seizures in these patients, Novartis run additional trials focused on this disease aspect and this is how everolimus obtained the marketing authorization as an orphan drug for treating epilepsy in patients with TSC in 2018, nine years after the initial orphan drug designation.
The story of stiripentol is quite different. Stiripentol (Diacomit, by Biocodex) had completed two clinical trials for treating Dravet syndrome and obtained orphan drug designations for treating this rare disease by the EMA and FDA in 2007 and 2008 respectively. The European agency granted stiripentol a conditional approval in 2007, which was later confirmed as regular marketing authorization, but the FDA did not approve the drug. For the next 10 years, stiripentol was in the market in Europe and Dravet syndrome patients in the US had difficulties to access it and have it reimbursed as a non-FDA approved drug. Then in 2018, after the 10-year European orphan market exclusivity had ended for stiripentol, the FDA finally approved it. In a way, this approval represents a regularization of the drug in the US market, while it had been already approved for that same indication in Europe for over a decade.
The two lines at the center of the graph with about 4 and half years of delay between orphan drug designation and marketing authorization are the two approvals of cannabidiol oral solution (Epidiolex, by Greenwich Biosciences)for Dravet syndrome and for Lennox-Gastaut syndrome, two rare epilepsy syndromes. The drug obtained the orphan drug designation of these syndromes in late 2013 and early 2014 respectively on the basis of early clinical data, and after completing two pivotal trials for each of these indications it obtained both marketing authorizations in 2018. The story of Epidiolex would be the usual one for a new drug that obtains the designation early in the development processand after 3-5 years completes its clinical development program and gets approved.
At the very right of the graph there is a drug with very short period between orphan drug designation and approval, less than 2 and a half years, which is more often the story of older molecules that get re-developed for an orphan indication. In this case the molecule is midazolam, a widely-used benzodiazepine. In February of 2016, Meridian Medical Technologies obtained the orphan drug designation for the use of midazolam (Seizalam) for treating status epilepticus in adults. The new product is a reformulation of an old molecule for an indication where it was already used, and the company obtained marketing authorization for the intramuscular delivery of the molecule. Because in this case a complete development program was not necessary, and because the orphan drug designation came rather late in the development process, the story of Sezalam is an usual one.
If you are still wondering about the drug that took 23 years between receiving an orphan drug designation and reaching the market, it is pegvaliase-pqpz (Palynziq, from BioMarin) for the treatment of Phenylketonuria (PKU). Palynziq is a recombinant protein and was granted the orphan drug designation for treating PKU in 1995. In the press release after approval, BioMarin’s CEO Jean-Jacques Bienaimé, highlighted how this approval represented “the culmination of more than a decade of perseverance by BioMarin employees”. An engagement with the FDA of over 20 years is indeed, a story of perseverance.
Ana Mingorance, PhD
Top 5 insights from the American Epilepsy Society meeting (2018)
Every year the American Epilepsy Society (AES) meeting gets larger. This year, over 6,000 people gathered in New Orleans to discuss the latest information about epilepsy care and the development of new treatments for epilepsy. The 2018 meeting captured the latest developments in the field of epilepsy drug development, where rare disease populations and new technologies are two areas of considerable growth and that are changing the way we will treat epilepsy. This article highlights what I found the most interesting at the AES 2018 meeting.
Every year the American Epilepsy Society (AES) meeting gets larger. This year, over 6,000 people gathered in New Orleans to discuss the latest information about epilepsy care and the development of new treatments for epilepsy.
I look for therapies for rare genetic epilepsies, so this biases some of my focus during the meeting. At the same time, many of the biggest developments have been precisely in the field of rare epilepsies, so this has been a very exciting year.
Here is the list of what I found the most interesting at the AES 2018 meeting:
1- Many new epilepsy drugs are orphan drugs
Probably the star of the AES 2018 meeting was GW Pharmaceuticals, operating in the US as Greenwich Biosciences, with Epidiolex (cannabidiol oral solution) now in the market for the treatment of epilepsy in Dravet syndrome and Lennox-Gastaut syndrome in the US. Greenwich had a very large presence at the meeting, with a prominent spot in the exhibition hall and the most crowded scientific exhibit. There was also a very popular session on the perspectives of physicians and patients about using cannabidiol for the treatment of epilepsy, which highlighted the interest of the patient and medical community on Epidiolex.
But there is more interest in the orphan epilepsy space than just Epidiolex. Next to Greenwich at the exhibition hall we could see Zogenix, with Fintepla (fenfuramine) about to file for an NDA for the treatment of epilepsy in Dravet syndrome, and BioMarin with Brineura (cerliponase alfa) for CLN2 disease, a type of Batten disease.
There were also other orphan epilepsy players that didn’t have a stand at the exhibition hall but had important presence at the AES meeting, most notably Marinus Pharmaceuticals which is currently in Phase 3 trials in CDKL5 Deficiency Disorder (CDD) with ganaxolone. Marinus had multiple poster and platform presentations, and a very well-attended scientific exhibit, showing early clinical data as well as biomarker data in CDD and PCDH19, to orphan epilepsy syndromes.
2- From symptoms to disease: epilepsy goes beyond pharmacology
There was one key progress visible at the AES 2018 meeting that defines a before and after moment in the field of epilepsy, and this is the arrival of non-pharmacological therapies for treating epilepsy.
Until now, we have seen progresses in many genetic epilepsies, using approaches such as enzyme replacement, antisense treatment or AAV-based gene therapy. But these were still not so visible in epilepsy, with the exception of Brineura for CLN2 disease which could be considered a neurodegenerative disease with epilepsy, more than an epilepsy syndrome. This year at AES 2018, however, we could see a broad range of disease-modifying experimental therapies in preclinical development for the treatment of different forms of epilepsy that are likely to lead to clinical trials using antisense approaches or viral gene delivery within two to three years:
Stoke Therapeutics presented some early but very impressive preclinical proof-of-concept data for their antisense oligonucleotide treatment for Dravet syndrome. The antisense treatment results in an increase in the Nav1.1 protein, and the company plans to initiate clinical trials in 2020 (see poster).
RogCon Biosciences and Ionis presented data on an antisense oligonucleotide treatment, this time for SCN2A epilepsy linked to gain-of-function mutations. The antisense treatment results in a decrease in the Nav1.2 protein and they showed efficacy in a mouse model (see poster).
A team by the Royal College of Surgeons in Ireland presented a very interesting approach, where they used antagomirs (which are also antisense oligonucleotides) to reduce the activity of miR-134 in a mouse model of epilepsy, leading to strong long-lasting seizure suppression. Interestingly, this approach would not be just targeted to a specific genetic epilepsy but might represent an alternative to pharmacological treatments or brain surgery for treating refractory epilepsy resulting from multiple (including unknown) causes.
A team from the Columbia University Medical Center also presented an approach for using viral delivery of a specific micro-RNA against the gene DNM1. This gene is mutated in an epilepsy syndrome, and the mutant alleles act have dominant negative properties. The approach, piloted in a mouse model of DNM1-dependent epileptic encephalopathy, improved multiple disease phenotypes.
Another surprise at the AES meeting was the first appearance of Encoded Genomics, still in stealth mode, as the sponsor of the Dravet Syndrome Roundtable. The Encoded team explained that the company is developing a gene therapy for Dravet syndrome, although no more details have been communicated at this point.
And although still using small molecules, Praxis Precision Medicine (1,2,3) and Xenon Pharma (1,2,3,4,5,6) also presented very interesting data of their Phase 1 programs to target specific genetic epilepsies caused by mutations in sodium and potassium channels, although they also have potential beyond these orphan epilepsies.
The number of programs in development using these new technologies, as well as the involvement of private companies in these programs, is unprecedented for the epilepsy field and make 2018 as the year when the new therapeutic approaches took a first important step in the epilepsy field. Within a few years we should see multiple of these disease-targeting programs in clinical trials.
3- Multiple great treatments for Dravet syndrome
Following the diagnosis of a child with a rare genetic disease, often families are told that the industry is not interested in developing treatments for them and that there is little research or that their disease is barely understood. This is definitely not the case for patients with Dravet syndrome.
There were 65 presentations on Dravet syndrome at the AES 2018 conference, and the syndrome is the target indication for some of the most promising pharmacological approaches in development as well as soon to reach the clinic disease-targeting approaches:
Epidiolex (cannabidiol oral solution) just got approved in 2018 in the US for treating epilepsy in Dravet syndrome (European decision expected in early 2019).
Fintepla (fenfluramine), from Zogenix, has completed two successful Phase 3 trials in Dravet syndrome with very impressive efficacy, and the main question mark around this dru,g which was a potential cardiac safety concern, has so far proven to be not a problem in this patient population. Fintepla is on track to be the next drug approved for treating this syndrome.
Ovid Therapeutics and Takedaare partnering around the development of TAK-935, a novel antiepileptic drug, which is also in clinical trials for Dravet syndrome. There were a poster and a talk on the preclinical proof-of-concept for this drug in a mouse model of Dravet syndrome and the data was extremely solid, with impressive efficacy in preventing spontaneous seizures and early mortality in the mice. So as impressive as Fintepla is in this population, TAK-935 might be a fair contender with a differentiated mechanism of action. Both therapies are also in clinical trials for Lennox-Gastaut syndrome.
Stoke Therapeutics follows the Ovid and Takeda collaboration in the pursuit of Dravet syndrome as the target indication for their lead antisense program, and also showed very good early data in a mouse model of Dravet syndrome at AES 2018. Their therapy will be the first disease-modifying approach to reach the clinic for Dravet syndrome after multiple pharmacological trials.
And as we wait for more information about the program, the gene therapy for Dravet syndrome in development by Encoded Genomics might follow Stoke’s antisense therapy into the clinic, completing a very promising pipeline of treatments in development for a single orphan epilepsy.
Possibly as a reflection of this increased industry interest in Dravet syndrome, the Epilepsy Therapy Screening Program run by the NINDS mainly at the University of Utah now offers a test in a genetic mouse model of Dravet syndrome that was also presented at AES 2018.
4- Near-approval treatments for acute repetitive seizures
There are some important progresses towards the management of acute repetitive seizures (cluster seizures) that were presented at AES 2018 and are worth highlighting.
A study presented at the conference highlighted that clusters (more than one seizure within 6 hours) were present in about half of pediatric patients with active epilepsy. Seizure clusters are common in many refractory epilepsy syndromes, and they are managed by using rescue (acute) medication. Rectal diazepam is the most widely use rescue medication for seizure clusters, but there is a strong demand from the patient community to develop alternatives. There are now two programs that would provide a suitable alternative and that were presented at AES 2018: intranasal diazepam (NRL-1, by Neurelis), and intranasal midazolam (USL261, by UCB Pharma). Both programs are expected to obtain the marketing authorization soon by the FDA. Their future in the European market is less clear given that the EMA has not yet accepted acute repetitive seizures as a separate orphan indication, which is the status of these experimental therapeutics at the FDA.
I also found interesting the description of a novel mouse model of acute repetitive seizures, which will support the development of new drugs beyond the currently used benzodiazepines.
Last, a young company called Engage Therapeutics is pursuing a very innovative EpiPen-like approach to try to abort seizures using a hand-held inhaler for fast systemic delivery of alprazolam. They announced at AES 2018 that they are starting a double-blind placebo controlled study.
5- AES is also a big meeting for patient organizations
A growing number of rare epilepsy patient organizations are using the AES annual meeting to host focused meetings with their main clinicians and scientists as well as with the companies developing therapies for that disease (or interested in doing so). Some of the veterans are the Dravet Syndrome Foundation and the Lennox-Gastaut Foundation, but we are also seeing more and more of the smaller groups, some of them in the first or second year of activity, also using AES to bring into a room the different stakeholders that are going to help them develop new treatments. In these focused meetings patient representatives and industry break their distances and learn from each other, becoming very educational and productive meetings.
Patient organizations were also present at the exhibit hall, and this year I counted 19 different groups.
Last, I really liked starting to see patient advocates take the stage as part of the main conference program. For example, I enjoyed listening to Dr Tracey Dixon-Salazar from the Lennox-Gastaut Foundation share the stage with some of the main neurologists who have run the Epidiolex clinical trials. In this type of medical conference, we should always ask ourselves: “medical specialists are telling us that this new drug has a favorable risk/benefit profile, but what do the patients think about it?”.
Summary
I had closed my review of the European Congress on Epilepsy (August 2018, Vienna) saying that “I personally missed hearing more about CDKL5 deficiency disorder and other “less popular” rare epilepsies, and about some of the non-pharmacological therapeutics in development, such as antisense therapies and gene therapy approaches.”
Looking back at those comments I’m glad to have seen more presentations about less popular rare epilepsies, including the multiple presentations by marinus on CDKL5 Deficiency Disorder, as well as the many disease-targeting approaches that I listed under section 2. In that sense I feel that the AES 2018 meeting captured better the actual developments in the field of epilepsy drug development, where rare disease populations and new technologies are two areas of considerable growth and that are changing the way we will treat epilepsy.
I can’t wait to see what 2019 has to bring!
Ana Mingorance, PhD
IMPATIENT SERIES #5 – EVALUATING AND TRACKING PROJECTS
When you let the scientific community know that your organization is open to fund research around your rare disease you are likely to get many research proposals from academic groups. These will come in different qualities, and will have different relevancefor your disease. This entry adds to Impatient Series I and to the ImpatientRevolution book and discusses in more detail how to determine that a proposed project is the right one and how to monitor its progress.
In the first entry of the Impatient Series I discussed what patient organizations mean when they say they are doing research.
This entry adds to Impatient Series I and to the ImpatientRevolution book and discusses in more detail how to determine that a proposed project is the right one and how to monitor its progress.
When you let the scientific community know that your organization is open to fund research around your rare disease you are likely to get many research proposals from academic groups. These will come in different qualities, and will have different relevancefor your disease.
As in the proverbial “if all you have is a hammer, everything looks like a nail”, most groups will try to tell you that the way to address your disease is to apply exactly the same techniques that you are using to your particular disease model. This is not an attempt to mislead you, since they all have chosen to build their laboratories around that specific technology or approach because they believe in it. This is why you need impartial advisors to help you select the best and more relevant applications.
HOW TO JUDGE THE QUALITY
The best way to determine the quality of research proposals is to use some trusted scientists as reviewers. Scientists are used to evaluating other scientists research proposals and manuscripts, it is part of their usual jobs.
Some of the aspects they should pay attention to are: does this group have the expertise that they need for this project? Are the proposing the right controls? Is the amount of work realistic with the proposed duration and funding request?
I also like to ask if this is the best lab that could do this approach, since a group might conclude (perhaps correctly) that the best approach for your disease is to develop a gene therapy and propose to do this in their lab, even though they are not a lab that has done gene therapy before.
HOW TO JUDGE THE RELEVANCE
It doesn’t matter how excellent the research proposals are, if they don’t directly advance your agenda you should not fund them. You did not create a Foundation to compete with the NIH for funding the very best grants, you created it to fill the gaps of your specific disease research field so that therapies can be developed faster.
Because of that your scientific advisors should also be able to evaluate if a given proposal is an interesting area to explore, vs a core bottleneck in the field to address, to cite to extremes.
To make the life of the applicants easier, I recommend that you decide, before opening a call for grants, which are the core interest areas that you are seeking proposals for. The more specific you can be the better. Saying that you are looking for proposals to “advance knowledge of the disease X, translational research or clinical research” would be too vague and of little help. Saying that you are looking for proposals to “identify substrates of kinase X, develop translational biomarkers and to validate new clinical outcome measures” would be much more clear.
Having pre-determined your key interest areas will also help you see if there is one of them for which you are not receiving proposals, in which case you might want to directly reach out to specialists in the area and propose them to work with you to address that need.
HAVING AN INTERNAL TEAM
At this point, if you are a young patient organization or foundation, you might be wondering how to manage the complexity of identifying scientific advisors, sourcing grant proposals, and determining research priorities. As I explained in the Impatient Series I entry, I am a strong advocate for you to consider having a researcher or an internal team, depending on your side, that works for your organization and that can be on top of all this. Having a researcher will also facilitate conversations with the industry and with regulators, since the right researchers will have a background that will make them capable of interacting with those professionals.
Having a scientist that works with you, even if part time, will also be very important for monitoring the progression of the projects that you are funding and making sure these are successful. In the next Impatient Series article I will discuss my thoughts about scientists working inside patient organizations or foundations.
MONITORING PROJECT PROGRESS
For monitoring project progression, I recommend quarterly project reviews (ideally written reports), and a final written report. Unless there is a big milestone coming up soon, requesting updates more often than that is not useful for research. Of course you should always have back and forth of short communications with the labs to make sure they all have what they need to move forward and be able to help with the troubleshooting.
It is a good practice when you follow up with the labs to ask “is there anything we could do to help you with this” whenever the lab expresses some delays or difficulties. This is also why it is useful to have a scientist doing these follow ups.
At the Spanish Dravet Syndrome Foundation we also used to run twice a year portfolio reviews, which were a half a day face-to-face meetings where every funded lab would present their results in the last period. At the Loulou Foundation we do this once a year, also for half a day, the day before our big CDKL5 Forum. The benefit of these meetings is that not only the Foundation gets an update about the projects, but all the different groups in your current portfolio get to see each other’s presentations and every single time that starts sharing and collaborations. I find these meetings very useful.
If you schedule these portfolio reviews right before another important meeting, for example your annual families meeting, and ask the groups to budget for this trip in their grant proposal, you will make it easier for them to also attend your meeting and potentially give an update to the families and other members of the community during the same trip.
At the end of the funding period, the groups should provide you with a financial update, outlining where the money was spent. For quarterly reports I think only a scientific update is needed.
Another important aspect to consider is intellectual property (IP). Some organizations don’t ask for any rights to part of the IP, while some others might ask for too much. I like very much the Loulou Foundation policy of asking for a percentage of the benefits from IP proportional to the funding that was used to create that IP. This empowers us to ask the groups to consider if any part of their discoveries is susceptible for IP creation, for example the method of use of an old drug for treating your disease if that is what they tested in their project. In this specific example, an academic group naive to how drug development works might publish the results without submitting a provisional patent application first, making it impossible for a company to then take that drug into clinical trials for your disease. Having the rights to part of the IP revenue also gives us a seat at the table during technology transfer negotiations, and help us see if the university is making enough efforts to get that IP turned into an actual product or drug program or if they are sitting on it and not making any efforts. To prevent this scenario, you might want to include some clawback provision into your intellectual policy clause which the groups need to sign in order to receive your funding.
Although asking for shared IP rights is attractive, you also have to be realistic. There are very few CureDuchenne. If you can only give small grants, you can’t expect to have shared IP. For example most patient groups I. know can only provide $20-50,000 per project, which is just co-funding the project. One the other hand, many of the Foundations are able to give significantly larger grants, of over $100,000 per year, and stand a more realistic chance of asking to have a percentage of the IP revenue. To know what you can ask, I recommend you seek advice from people familiar with technology transfer who will be able to advice you based on the particularities of your organization.
Last, make sure you request your grantees to notify you of any publications and conference presentations that result from the project that you fund, and that they include your funding in the acknowledgments. This will help you track the results of your funding beyond the duration of the funding period.
The next entry of the Impatient Series will address how to find a scientist to work with your organization.
Let’s start an #ImpatientRevolution!
Ana Mingorance, PhD
Main lessons from the 2018 CDKL5 Forum
For the past four years the Loulou Foundation hosts an annual “by invitation only” meeting where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances. This was the second Forum I attended, and my first since joining the Loulou Foundation.
Here are the main news and take-home messages from the 2018 CDKL5 Forum that took place in London, UK, in October 22 and 23.
For the past four years the Loulou Foundation hosts an annual “by invitation only” meeting where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances. This was the second Forum I attended, and my first since joining the Loulou Foundation.
There were major news announced during the meeting, like a new Phase 2 clinical trial in CDKL5 deficiency disorder with fenfluramine, a drug that has completed two Phase 3 clinical trials in Dravet syndrome with very strong efficacy, and the announcement that Ultragenyx will develop a gene therapy for CDKL5 deficiency disorder.
For those of you who didn’t attend but are interested in the field, here are the main news and take-home messages from the 2018 CDKL5 Forum that took place in London, UK, in October 22 and 23:
1. The development of therapies for CDKL5 deficiency disorder has progressed very fast
One of the CDKL5 researchers explained from the stage that 4 years ago there were only 20 publications on CDKL5. Four years later, he was standing in a room with nearly 200 scientists and drug developers, discussing 4 clinical trials. From what we know today about the pipeline, in 4 more years we might already have the first symptomatic drugs approved, and will be running clinical trials with the enzyme replacement therapy and gene therapy that are already in development. This speed of development in a rare disease is truly remarkable.
The Chief medical Officer of Marinus echoed these comments by reminding the audience that just a year ago they stood in front of the regulators discussing if “CDKL5 deficiency disorder” was truly a disease or simply a gene that can present as many different syndromes when mutated. After the Loulou Foundation and the patient organizations mobilized and made sure that CDKL5 deficiency disorder was listed in the major disease classification websites and documented as the separate entity that it is, the FDA not only accepted the indication but also approved the first pivotal trial for this disease. We went from not having a recognized disorder to getting green light for a pivotal trial in a matter of weeks.
2. There is a strong industry interest in CDKL5 deficiency disorder
In addition to Marinus Therapeutics, which is in the middle of recruiting for their Pivotal stage Phase 3 trial for CDKL5 deficiency disorder, there were 22 more companies in the room at the 2018 CDKL5 Forum. Last year meeting took place in Boston, and 35 companies attended, but I personally through that the majority would only be there because they have local offices and it was easy for anyone who was just mildly curious to drop by. Having 23 companies come to London means the interest goes beyond mere opportunistic curiosity, and that list of programs in development to treat CDKL5 deficiency disorder is likely to grow in the next few years as this interest materializes in development programs.
The Loulou Foundation honored the companies Takeda and Ovid Therapeutics during the Forum in recognition of their contribution to research and therapeutic development in CDKL5 deficiency disorder
The current pipeline for CDKL5 deficiency disorder has one drug in Phase 3 (pivotal) trials, and three drugs in Phase 2 (proof-of-concept) clinical trials:
Phase 3: Ganaxolone, from Marinus Therapeutics, currently enrolling through 40 clinical sites across EU and US. This is the only placebo-controlled pivotal trial requested for registration of the drug, so once completed Marinus should be able to file for marketing authorization. The company estimates to enroll 70 to 100 patients in total.
Phase 2: Ataluren, from PTC Therapeutics, currently completing a placebo-controlled investigator-initiated study at NYU Langone Medical Center in children with CDKL5 deficiency disorder caused by non-sense mutations. This study enrolled 9 patients.
Phase 2: OV935 (TAK-935), from Ovid Therapeutics in partnership with Takeda, currently enrolling for an open-label study through multiple sites in the US a total of 15 patients with CDKL5 deficiency disorder.
Phase 2: Fenfluramine, from Zogenix, soon to start enrolling for an open-label investigator-initiated study at NYU Langone Medical Center in children with CDKL5 deficiency disorder. This study will enroll 10 patients, and was announced for the first time at the CDKL5 Forum.
3. Companies developing cure-like treatments for CDKL5 deficiency disorder are moving forward aggressively
Amicus recently announced a collaboration around a new AAV (gene therapy)-based technology to complement their enzyme-replacement therapy in development for CDKL5 deficiency disorder. With this new approach, they will use a virus to deliver a secretable form of the CDKL5 enzyme to brain of patients, so it can replace the missing endogenous enzyme.
The first day of the 2018 CDKL5 Forum meeting brought the groundbreaking news that Ultragenyx, one of the strongest players in the rare disease space, had reached an agreement with RegenXBio, one of the strongest players in gene therapy development, to develop a gene therapy approach for CDKL5 deficiency disorder.
With the strong interest that this rare disease is attracting, and its potential tractability by enzyme replacement and gene therapy approaches, we are likely to see the number of companies working in this space grow, and hopefully some clinical trials starting in a couple of years.
4. The preclinical knowledge and preclinical toolbox have progressed much
We have progressed much in our understanding of the biology of CDKL5 and the consequences of the deficiency for cells and animals. One of the main breakthroughs of the past year has been the identification of some of the substrates of CDKL5, which were discovered in separate labs using different approaches and are therefore very solid findings. Interestingly, these key phosphorylation targets are cytoskeleton-binding proteins, which appears to be one of the main cellular domains where the kinase is important.
It was also encouraging to see that there are multiple mouse models generated for CDKL5 deficiency disorder, either missing the gene or carrying patient mutations, and that there are some solid phenotypes that are reproducible across labs and that can help carry out preclinical trials in the disorder. Interestingly, these mice don’t develop spontaneous seizures, but with four drugs in clinical trials for the disease using seizures as the primary efficacy endpoint this does not seem to be a problem for the pharmaceutical industry. The consensus in the room was that “a mouse is a mouse” and as long as the mice have clear neurological phenotypes that are driven by the deficiency in CDKL5 they are good models. The most anticipated experiment, the “rescue” experiment where expression of CDKL5 is turned back on in previously deficient mice, is about to start thanks to the creation of a conditional mouse model and the entire field awaits with expectation those results.
5. Getting the field ready for more complex clinical trials
We also discussed during the Forum that although the first clinical trials in CDKL5 deficiency disorder are using seizure frequency as the primary efficacy endpoint, it is expected that future clinicals will measure more broadly the developmental disability of the disease and any potential improvement from treatment. Speakers from Ultragenyx and Roche explained how the industry approaches the development of new clinical outcomes and the importance of precompetitive collaborations in this space to move the field forward. The Loulou Foundation and IFCR (the US CDKL5 deficiency patient organization) are planning to host an externally-led PFDD meeting with the FDA in 2019 that will also assist with the identification of new meaningful clinical outcomes.
A captivating and inspirational Dr Emil Kakkis, from Ultragenyx and the EveryLifeFoundation, shared with the audience his journey to convince the industry that developing therapeutics for rare diseases was worth it, and some of his victories and approaches to turn these aspirations into a reality. He made a call for the use of multi-domain outcomes and biomarkers in clinical trials in rare populations, and why he thinks that the golden age of rare disease treatments is now coming to CDKL5 deficiency disorder.
“These are exciting times for rare diseases, and CDKL5 deficiency disorder is now front and center. Collaboration and cooperation is needed to identify the best outcomes as soon as possible. “
6. There is a strong patient community behind CDKL5 deficiency disorder
The very young CDKL5 Alliance, the umbrella organization that groups 15 patient organizations and foundations, announced during the CDKL5 Forum the launch of their website with the hope of making it easier for any family around the globe to stay updated about progresses in the field and to connect with other patient families (http://www.cdkl5alliance.org).
The CDKL5 Alliance met during the Forum and set some of their next priorities, including the identification of more patients and setting Centers of Excellence in every country so that the community is ready to run multiple clinical trials. They also receive the Champion of Progress 2018 award from the Loulou Foundation for building and keeping the community together.
During the Forum, the patient groups and the Loulou Foundation were credited with having been instrumental to the large progress in the field so far. From providing the key initial funding to research labs, to helping create Centers of Excellence and reaching out to companies to encourage them to consider their disorder and help them. Essentially, the secret for points 1 through 5 in this summary is the strong patient community behind CDKL5 deficiency disorder.
The patient groups and the Loulou Foundation have also managed to createa broader community where scientists and drug developers also find their home. This was particularly visible during the 2018 CDKL5 Forum where drug developers mixed with academic scientists and clinicals as speakers and moderators in the meeting, and where breakout sessions to help design the future of CDKL5 research sat around the table researchers, drug developers and patient parents without distinctions.
All in all this was a really good scientific meeting, with strong collaboration from all sectors in the CDKL5 community, and where it became clear that as Dr Kakkis said CDKL5 deficiency disorder is now front and center, and where we have a real opportunity to change the future of the disease.
Ana Mingorance, PhD
Impatient series #4 – The place for patients in preclinical research
Once there is a drug that has been developed, it is very clear why talking with patients and collaborating with them is useful for pharmaceutical companies. What is less obvious to both companies and patient organizations is how patients can be active in research before there is any drug in development, during the preclinical phase, and how this might lead to medicines being developed. Some patient organizations might start by funding some academic group, but without a clear vision and a longer-term strategy the return on those efforts will be compromised, and medicines will take longer to come. Here I want to outline the broader strategy that patient organizations can follow to advance research towards new medicines even before companies are working on it.
Once there is a drug that has been developed, it is very clear why talking with patients and collaborating with them is useful for pharmaceutical companies. They often learn about the disease from patients, so that they can better understand the symptoms and how to design clinical trials. Patients are also involved at different stages in the regulatory pathway, providing feedback to the regulatory agencies during trial protocol approval, orphan drug designation or marketing authorization.
What is less obvious to both companies and patient organizations is how patients can be active in research before there is any drug in development, during the preclinical phase, and how this might lead to medicines being developed. “Preclinical” refers to the research that is done before a therapy can be tested in patients. Some patient organizations might start by funding some academic group, but without a clear vision and a longer-term strategy the return on those efforts will be compromised, and medicines will take longer to come.
What follows is my advice on how to approach early-stage research for those interested in advancing research from a patient organization. My main advice to patients working on a disease is to “own” the field, don’t leave this up to academic scientists.
To focus on advancing the research field means more than funding a couple of research programs. I list many specific research programs that are important early on in a field in the eBook the #ImpatientRevolution. Here I want to outline the broader strategy that patient organizations can follow to advance research towards new medicines even before companies are working on it.
1) CONNECT
A key value that patient organizations bring to a research field is to create communication channels between the different researchers working on it. This might be formalized, like hosting an annual scientific meeting, or more informal, simply by knowing everyone in the field and introducing them to one another. By creating these connections researchers start collaborating and have improved ability to obtain funding as they bring key collaborators onboard. So no only research will be improved by increased communication, but also this community will be able to mobilize more public funding towards their rare disease by building a stronger scientific base. The way to start these connections can be as simple as reaching out to corresponding authors in PubMed publications related to your rare disease, and by always asking “who else do you recommend me to talk to?”.
2) TOOLS
For scientists to work on a rare disease, or any disease in general, they need to have a tool box. These are research tools, without which scientists cannot build the science. Research tools include animal models of the disease, cell lines (lab cells expressing the disease protein or patient-derived cells), antibodies that recognize the disease protein, or the DNA of the disease gene that is then used to create these models. For anyone to research a rare disease, they are going to need to use these tools.
Because they are so necessary, scientists who are the first to invest the time and resources to create those tools might hold on to them, limiting who else can work in the field. This happens very often in rare diseases, when only one lab has a knockout mouse model for the disease, or has the only good antibody for that protein. Keep in mind that academic science is very competitive, and being the only lab in the world able to do something is highly prized. This creates the incentive of not sharing, and seeing other labs wanting to work on the same disease as competitors.
Don’t let any scientist decide who can or cannot do research in your disease. Build all these tools yourself and make them open-access – or if you finance a lab to build these tools make sure your funding contract makes it mandatory for them to share it with anyone afterwards, for-profit companies included, without being able to veto anyone.
By making sure your rare disease has the complete toolbox available to all scientists you will mobilize a large number of researchers towards your disease. You might also start to attract pharma companies. Reducing the barriers of entry to your field is one of the best things you can do as a patient organization.
3) KEY QUESTIONS
Beyond investing in building the toolbox, you need to identify which are the key questions around your specific rare disease that might also represent a barrier of entry for researchers and pharma companies. It might be that the incidence of the disease is unknown because the first handful of patients has just been described. It might be that the disease is caused by a mutated kinase, and the targets of this kinase are unknown. It might be that the rare disease involves the deletion of a chromosome fragment involving dozens of genes, and the individual gene(s) among all these which is responsible for the disease is unknown. These are all some examples of important questions that are important to solve before therapies can be developed for this disease. If these are still unanswered, as a patient group these should rank very high in your priority list.
I recommend targeting these questions by approaching labs that have already solve this type of questions, even if they are not yet interested in your disease. They are much more likely to be able to complete these projects successfully than labs that you might already be in contact with, who are already familiar with your disease, but who lack the core expertise to ask these questions. You don’t have the time to wait around labs developing new skills, you should try to put together the research tool box and solve the key questions as soon as you can so that a large group of scientists (and potentially pharmaceutical companies) start working on developing therapies for your disease.
4) FAST MEDICINES
Remember the importance of developing the toolbox? One of the early uses of having cell lines and animal models for your rare disease is to be able to find a new drug the fastest way possible: by finding a new use for an old drug.
You will most likely hear early into your journey about drug repurposing. Drug repurposing refers to finding a new use (a new purpose) for an already existing medication, which enables doctors to use it to treat this new disease as well. In some cases there will be clinical trials to seek approval for using that drug in the new disease. In most cases there won’t be clinical trials, and doctors will simply prescribe it off label. In either case as a patient group one of the earliest questions that you should aim to answer is whether there is a drug already out there, sitting at the pharmacy shelf, that could potentially treat your disease. The best way to do this is by using cell lines expressing your disease protein or gene, or by using small animal models such as yeast, c. elegans or zebrafish (the choice depends on the particular rare disease), and use them to run a screen of approved drugs, looking for those able to restore protein function or address a particular disease phenotype. Running a repurposing screen to identify any potential drug that is already available should also very high in your priority list.
A 2.0 version of these efforts is to use those cell lines or animal models to evaluate drugs already in clinical development for related diseases. If you identify a drug with efficacy, the company that is developing it might be interested in expanding those clinical trials to include patients with your disease.
That’s why it is so important to consider all the drugs already developed (or near approval), because if you find a drug that is already approved or in clinical trials and that has efficacy in your disease you will be cutting down by many years, potentially a decade, the wait for new medicines.
5) ENGAGE PHARMA – EVEN AT THE PRECLINICAL STAGE
Last, I still recommend you to try to engage with the biotech and pharma industry as early as you can. Ask them about how they see your disease. Do they see it as similar to another disease? Perhaps similar to a large disease? Do they flag some immediate research gaps? This feedback will help you shape your research strategy as you build a relationship with the industry and learn to think about therapy development.
But these conversations are often not happening, or are not happening early enough. The reasons are multiple on both fronts:
What patient organizations often want when collaborating with companies before there are drugs in clinical trials
Most patient organizations are in touch with companies that have a drug in clinical trials for their disease. Patient organizations that approach companies before these companies have a drug so advanced are often interested in trying to convince the company to work on their disease. Because in most cases this will simply not happen, it is best if the patient groups can switch their focus to learning to understand how industry scientists think about diseases and get their input about their rare disease field. They can also be good door openers if they know people in other companies.
What companies often want when collaborating with patient organizations before there are drugs in clinical trials
At early stages, before they have a drug in development for that disease, industry scientists are interested in learning about a disease beyond what is already published in the medical literature. They want to know what the incidence of the disease is, if there are centers of excellence, and many of the questions I covered in the “when is your disease field ready to attract the interest of companies” article earlier in this Impatient Series.
What holds patient organizations back from collaborating with companies before there are drugs in clinical trials
Patient organizations sometimes refuse to engage with pharmaceutical companies because they believe it creates a (perceived) conflict of interest. They think it is better to remain on the academic science side, and not collaborate with companies. Academic scientists don’t think the same way that industry scientists, and don’t have the same incentives (publications vs drugs approved), so it is a mistake to believe that academic scientists can replace the work and input of industry specialists. My advice is to avoid perceived conflict of interest or biases by simply engaging with every single company in your disease field, without picking favorites, and to make sure they have as many information as they need (see previous section).
What holds drug development companies back from collaborating with patient organizations before there are drugs in clinical trials
People working at pharmaceutical companies usually express the same two fears that keep them away from engaging patient organizations early in the preclinical stage or even before they decide to start a program in a disease. One is disappointing patients. They think that by talking to a patient organization, patients and their families will think that this company will develop a drug for their disease, be successful and reach the market. Industry scientists know that most programs will not even reach clinical trials, so they are worried about disappointing patients. I find it interesting that when I have talked to patients about this they often tell me that talking to companies and knowing that companies are interested in their disease makes them feel more at peace, versus worrying that no one is doing research or developing a drug. Living with a rare disease is hard enough, patient don’t think there is going to be a perfect cure developed overnight, they rather express the need to know that efforts are ongoing and that they are not alone at trying to look for a medicine.
The second main fear that industry employees express is lack of confidentiality. I will want to address this in a future entry in the Impatient Series. It is important for patient organizations to remain professional, and not disclose confidential information when they have access to it. If they do, they will stop knowing about things before the news are public and they will not be able to influence important processes like clinical trial protocol design early enough to make an impact.
IN SUMMARY, WHAT I RECOMMEND BASED ON MY EXPERIENCE
- Own the field. Be a field expert, not just a patient expert.You will do this by knowing everybody, putting together the room where conversations happen and having developed (or made available) the research tools.
- Be professional about it.Get advice from professionals, get advice from the industry, and if you are willing to invest funds in research then get professionals onboard that help you manage that.
- Collaborate with everyone – or at least talk to everyone - if they are professional about it. Don´t marry a lab, don’t avoid companies to remain free of conflict of interest. Ask to be treated with respect, for example by getting some feedback or update after you have met with a company to provide feedback on some of their work.
Let’s start an #ImpatientRevolution!
Ana Mingorance, PhD
Impatient series #3 – When is your disease field ready to attract the interest of companies
In the previous entry we discussed the many types of companies that get interested in rare diseases, each of them because of different reasons. If your disease field matches one of those business propositions you will get at the top of the list of interesting diseases for that company, but another part of the decision equation is time, or field maturity. Is this field ready for us to start working on it already The following are the questions that a company will often need to answer to be able to judge if the field is ready for them, too early for them, or maybe even too mature for them to get in.
In the previous entry we discussed the many types of companies that get interested in rare diseases, each of them because of different reasons. If your disease field matches one of those business propositions you will get at the top of the list of interesting diseases for that company, but another part of the decision equation is time, or field maturity. Is this field ready for us to start working on it already?
The following are the questions that a company will often need to answer to be able to judge if the field is ready for them, too early for them, or maybe even too mature for them to get in. Some of it applies to both large and rare diseases while some of the questions are more important for rare diseases.
If you work at a rare disease patient organization or research foundation, make sure you also ask yourself these questions:
o How many patients are out there? Some companies will be interested in diseases with as little as 500 patients, but others might have a cut off of 3,000-5,000 treatable patients. If the numbers are not known, or if they are too small, the probability of getting a company interested will be much much smaller.
o Is there already a drug approved that treats this disease in a satisfactory way? If a company can be the first-to-market it will be more interested in the disease.
o Who else is developing drugs for this same rare disease? The larger rare diseases might support competition, with multiple companies hoping to get a piece of the market, but if the disease is very rare one of the main drivers of companies to choose a rare disease, which is avoiding competition, will not make sense anymore.
o Do we know the cause of the disease and what is affected? This will help them see if your disease is a good match for their drug or technology, and even to see if they might have already have some already developed drug that could help your disease because it acts on the same signaling pathway.
o Are there good animal models of the diseases? Generally this is a mouse model. If there are good mouse models the company could either quickly test their drug to see if it has efficacy (if the drug already exists), or use it to confirm the efficacy of a future drug that they might want to develop. If there is no mouse model yet, some companies might worry that the disease might turn out to not be easy to model in a mouse, and that will make it harder to validate their drug in a relevant model prior to clinical trials. In some diseases, other animals are used to model the disease. What matters is if there is a good animal model that has symptoms in common with the patients or not, and if the company can have access to it.
o Are there other preclinical models available? The good mouse model is the bare minimum, for many research programs they will need to have access to DNA carrying the mutation and to cell cultures from patients.
o Is the patient population homogenous? Is there well-documented natural history? This is important to know which are the symptoms that need to be treated and to reduce patient variability. Patient variability is one of the problems of larger common diseases, and the reason of many trials failures. Therefore having patients that are very homogenous (diseases caused by one gene, for example) is a major attractive point of rare diseases.
o Could we find clinical centers willing to run clinical trials and patients willing to enrol? If there are too few specialists or they are not confident that they could find patients, companies will wait for the field to be ready.
o Would we know how to design a clinical trial? Depending on the disease symptoms it might be easy or not to know what could be measured during clinical trials to investigate drug efficacy. Not having a clear symptom to measure in a trial, what is generally called “an endpoint”, can be a major cause of clinical trial failure.
o Is there any previous case of regulatory agreement or approval? If the regulatory authorities (generally FDA or EMA) have already agreed with another company which endpoints are good, and how many patients are needed, and confirmed that they consider that rare disease a legitimate separate disease, then a lot of the regulatory risk has been lifted and more companies will be interested.
Because it takes time to be able to answer all of these questions in an affirmative way, some of the most recently described rare diseases will not be mature enough for companies to work on them. The next entry of the Impatient Series will address how, from a patient organization, you can advance research in your disease to the point and make it mature.
Let’s start an #ImpatientRevolution!
Ana Mingorance, PhD
Impatient series #2 – What are companies looking for when choosing a rare disease
The number of orphan products in development keeps growing every year. A particular rare disease will be attractive for a drug development company if it matches one of four main business propositions determined by the interplay of market pressure and technology enablers. The degree of maturity of a field will also attract companies and help prioritize which disease to focus on where multiple rare diseases match the needs of the company or the drug that they are already developing
As part of these Series I will write about what patient organizations can do towards advancing research, even before a company gets interested in their disease. But before I can address that, we need to first define what gets companies interested in a disease in particular. That will then serve as the goal for patient groups: get your disease field to the point where it can attract companies.
As a side note, some diseases will be so rare that they won’t be able to attract companies. In those cases, if patient organizations need to carry out all of the research and development activities, they will still need a field that is mature enough to support drug development efforts.
I will address this in two parts: first, why companies get interested in rare diseases, and second, why do they choose one rare disease vs another.
PART 1 – CHOOSING ORPHAN
The number of orphan products in development keeps growing every year. Therapies are called orphan when they target a rare disease, which were traditionally orphan in the sense of not having any medicine to treat them. But nowadays some rare diseases have multiple “orphan” drugs to treat them, such as cystic fibrosis, and they are still called orphan drugs.
Companies have been driven towards rare diseases for a combination of reasons. The main ones are probably a market push (away from large markets) and a technology pull (towards rare diseases):
A market push: some of the larger markets got so crowded that it became very difficult for companies to get a competitive price. This has been called the “better than the Beatles” problem. Imagine if every new musician would need to show that they are better than the Beatles in order to get a record out – this is what new drugs must demonstrate in order to get approved and be successful in the market. The rare disease space, however, represents a broad landscape of virgin diseases, for which no drug (or Beatles' song) has ever been approved, and where an effective and safe drug will be commercially rewarded.
A technology pull: with the development of new technologies, particularly genomics, we have been able to understand that what we thought were common diseases are in fact a collection of separate (genetic) rare diseases. Other technologies such as gene therapy and antisense therapy have also enabled us to treat these diseases, in a way that we could have never imagined some years ago.
Drugs developed to treat rare diseases also enjoy of some regulatory and market incentives, which were developed by the regulatory agencies to attract drug developers to this space. The most valuable one is probably the 7 to 10 years (US vs EU) of market exclusivity after approval during which no competitor can have a similar drug approved for the same disease (a generic). But this aspect is less important for patient organizations. Instead we should try to understand how the market and the technology might drive a company to our rare disease.
Looking at the interaction between the market push and the technology pull we can differentiate 4 types of companies that get interested in rare diseases or 4 types of programs within a company that might be directed towards a rare disease:
1- Companies that want large markets and use old technologies: These are your classical large pharma companies, that have the pockets that are needed to pay for clinical development in large diseases, and that are developing a traditional drug, usually a small molecule that can improve an important symptom. These companies have transformed the way we treat common diseases like diabetes or cardiovascular diseases and still produce the same type of drug, but today the problem is the market push that scares them away for the large diseases because there is too much competition and the requirements for pricing and reimbursement are too high. This is how a company that had a drug perfectly good for a large market, and that prefers large markets, finds itself developing their molecule for a rare disease that has that common symptom in order to avoid the competition.
2- Companies that want small markets and use old technologies: Some companies develop the same type of molecule that could treat common diseases but have decided to focus on rare diseases as part of their business model. This is usually the case of smaller companies, which don’t have sufficient money to go after the common diseases. Smaller companies often focus on smaller diseases because those are the ones where they can afford to take a drug all the way through clinical trials and into the market. A second type is when the molecule is an old drug. In these cases the orphan drug market exclusivity that I described before becomes very important and makes the company focus on a rare disease so that they can have product protection (since they don’t have a patent on the drug because it is an old drug). These companies focus on rare diseases because it is cheaper to take orphan drugs to the market and because of the market exclusivity.
3- Companies that want large markets and use new technologies: Some of the large companies, which like to go after large diseases, are also adopting new technologies. This allows them to go straight to the cause of the disease, in what we could consider personalized medicine. In these cases where the therapy targets a disease cause, not just a symptom, rare diseases that share that cause become very attractive as part of the drug development pathway. Imagine for example a company that wants to develop a medicine for Alzheimer's disease by creating a molecule that targets the protein “tau”, which is very important in Alzheimer's. Such company knows that most trials in Alzheimer´s disease fail, and that they cost many hundreds of millions of dollars. So such company will most likely choose to first test their drug in some monogenic taupathy (i.e. a rare disease caused by a problem in tau) to make sure that the drug does what it should, before risking costly Alzheimer’s disease trials. The rare disease becomes a stepping stone in the development program. This is another scenario in which a company that is going after a large market finds itself developing their molecule for a rare disease in order to de-risk their program.
4- Companies that want small markets and use new technologies: And last we have the companies that are focused around a technology platform that usually means they can target genetic causes. These companies working on new technologies such as gene therapy and other personalized-medicine approaches work on rare diseases because these are largely caused by genetic problems, so they are the perfect match for companies (large and small) that are using the new technologies. These are the companies most likely to specifically develop from scratch a therapy ideally designed for that rare disease, and to target the cause. These are the companies (and the business scenario) most likely to lead to the type of treatments that we could call cures.
Some diseases become popular for only of these business reasons. Others fit multiple boxes. It is important for each rare disease patient organization to identify which of these boxes they fit, so that they know how to target their efforts and conversations.
PART 2 – CHOOSING ONE SPECIFIC DISEASE
Q1 - Does it fit our business needs?
Your rare disease will be attractive for a drug development company if it matches one of the four main business propositions (previous graph):
1 - My drug discovery expertise is in epilepsy. The field of epilepsy got very crowded, with over 30 drugs approved, so pretty much every company in the sector started looking at the orphan epilepsies to avoid the competition. Except for some cases, most of their drugs are molecules that could have easily treated epilepsy in general, since they just treat the symptom (seizures) and not one of the many epilepsy causes. But the companies chose to work on the orphan space to avoid competition. That is how rare syndromes with epilepsy “got lucky”. Prader-Willi might be an attractive target for a company that is concerned about competition in the obesity market. Neurodegeneration is another field where companies are moving towards rare diseases, in this case due to the high failure rate in the larger diseases. If your disease has a common symptom and the general market is saturated it might attract these companies.
2 - A company with an old molecule will most likely be looking for a rare disease due to the need of securing some years of market exclusivity in the absence of a drug patent. Often this happens when the company has discovered a new property that the old drug has, which creates the possibility of developing for that new use. In this case they will be looking for a rare disease that matches that new use, either due to its cause or due to its symptoms. Also, some small companies developing molecules that could treat broad diseases choose to focus on the orphan space because they can't afford the large and lengthy trials that broader diseases demand. One example could be the multiple small companies going after autism, which often decide to target genetic syndromes with autism even if their drug is not specific for the syndrome.
3 - The large company that is targeting a molecular cause of a large disease will most likely be interested in identifying a rare disease that results from that same cause. Some diseases become quite popular as monogenic examples of large diseases. I am a neuroscientist, so I often use taupathies as an example of stepping stone. Gaucher disease also shares biology with some forms of Parkinson, so companies targeting glucocerebrosidase for the treatment of Parkinson’s disease are likely to run their trials first in Gaucher’s disease and only risk moving forward with Parkinson if the drug has good results. There are examples of this strategy in all disease areas.
4 - Companies or research programs that are built around a platform technology often have a series of requirements that the target disease must meet in order to be a good match for the technology. A good example is a great young company called Stoke Therapeutics, which has discovered a way to increase protein levels by targeting a step between the gene sequence and the protein production (excuse my vague descriptions for the sake of non-expert understanding). Because of their particular technology, Stoke is interested in diseases that couldn’t be treated with gene therapy (maybe because the gene is too large for “traditional” gene therapy), that is also not amenable to protein replacement approaches (so the protein cannot be a soluble enzyme, for example), that needs more levels of a given protein (and not less levels) and that has one good copy of the gene for their therapy to act on. Each technology will impose a different set of requirements.
It is important to remember that the same disease might be a good match for one of these technologies AND be mechanistically linked with a broader disease so it can be a stepping stone AND have some common symptom that makes it attractive for the opportunistic companies. In other words, each company might be interested in your rare disease for a whole different reason!
Q2 - Is the field already mature?
The previous question asked whether a particular company should be interested in your disease. The question about field maturity essentially asks if they should be interested in your disease NOW. It will also help prioritize which disease to focus on where multiple rare diseases match the needs of the company or the drug that they are already developing.
We will discuss this in the next entry of the Impatient Series.
In the meantime, let’s start an #ImpatientRevolution!
Ana Mingorance, PhD
Impatient series #1 – What patient organizations mean when they say they are doing research
Most patient organizations, no matter how small, start with the same mission: to raise awareness and funds for research in their disease. But what research are they doing? are they all starting in the same place? and do they all evolve to run the same type of research? In my experience there are many types of strategy and approaches that patient groups take, in particular when they are starting. What a patient organization means when they say “we are doing research”, therefore, hides many different possibilities. The article describes some of these types, and discusses their advantages and disadvantages.
When we could only look at symptoms, there were less diseases. Now that we can also look inside a cell and see specifically what went wrong, we are uncovering new diseases, thousands of them. This means that for a lot of people, the disease that they have or that their child has is quite new to science. So in the true spirit of impatient patients, some of those patients come together and start a patient organization.
Most patient organizations, no matter how small, start with the same mission: to raise awareness and funds for research in their disease.
But what research are they doing? are they all starting in the same place? and do they all evolve to run the same type of research?
In my experience there are many types of strategy and approaches that patient groups take, in particular when they are starting. Although no two are the same, they do fall overall into some big types. To make things more complicated, what a patient organization funds in year 1 has often little to do with what they fund some years later. The field has evolved by then, the organization has evolved, the board members might be different… What a patient organization means when they say “we are doing research”, therefore, hides many different possibilities.
Here are some of the strategies that I’ve seen:
Type 1: We just need to exist
There is a tricky catch-22 situation where in order to raise funds (which you would hope to use for research) you have to already show you are a successful patient organization that funds research. Beyond friends and family, you will have to show most other donors where their money is going and how much of an impact it is making. Essentially: you need to be already funding research.
Because of this, your first few projects are critical to show the world that your patient organization exists and that it funds research. The quality of what you fund is in fact secondary. In many cases the project(s) take place at a university located near the headquarters of the patient organization. This might get you into some local press release, provide you with a scientist to refer to when you need some interviews, and essentially get your ticket punched. Congratulations, you now have a patient organization that does research, and from there you can start building a more solid longer-term strategy.
Type 2: Marry a lab
The first researcher that approaches a patient group, in particular if it is a newly-described rare disease, sometimes goes on to become their official lab. As in the previous case, this often happens with organizations that are just starting off. For the next couple of years, the organization will raise funds to finance research at that main lab, which then becomes a referent in the field for that disease. These patient groups appear to be married to that initial lab.
Getting funding for academic research as an academic lab head is very hard. And if it is a new research area for that lab, it might be impossible. So, for some academic labs, receiving funding from those patient groups is the only way to advance their science during the first couple of years until they have generated enough data and publications about the new disease to be able to receive public funding. In a way, the patient group didn’t marry the lab, instead they adopted the lab until it was mature enough to become financially independent.
This is a good strategy when the patient group doesn’t have enough funds to finance multiple projects or labs and when the field is just starting. In this case, the patient group might choose to focus its resources on building a first solid lab, which will pretty much start building the entire research field. This is how some patient groups are doing research.
This strategy, however, can backfire in many ways. One of such scenarios is when the patient group leadership comes to believe that they should not fund other labs because they owe to this initial lab some loyalty in return for their early support. It blurs the line between grantee and patient group and could lead to big conflicts among the patient group leaders. Another potential negative outcome is not having much to show for after supporting the same lab for several years. This happens when the lab learns to see the patient’s money as easy money and not dedicate enough efforts to the project to make an actual tangible progress, unlike they would do for regular external funding.
In short: it is a respectable strategy to adopt one lab and start building a field, but it must be done with a clear outcome in mind, which is agreeing with the lab that they should dedicate those early efforts towards generating compelling data to become financially independent from the patient group. It must to be bridge funding, and not funding for life.
Type 3: Let the advisors decide
When patient organizations have enough funding to support multiple programs, they often choose to surround themselves with a handful of experts that will become their scientific and medical advisors. In many cases, they ask these advisors to tell them where to invest their research funds.
Often this is done by having an annual call for grant applications and then having the advisors and some external experts that they nominate evaluate the grants and rank them. Then the patient group will fund the top ranked proposals. This is how some patient groups are doing research.
One thing I don’t like about this strategy is that it is often bottom-up, with the external scientific community proposing what they would like to do and the patient group then picking the most scientifically excellent ones. I believe research funding should be more strategic and therefore more top-down, but there are ways to achieve a healthy balance.
My main concern with the advisors deciding where to invest is that in some cases a patient group ends up funding every year the same 4 or 5 labs, which happen to be the labs of their scientific and medical advisors and/or their close collaborators. If one can guess who your advisors are by seeing who you fund, you are an organization that has essentially followed the Type 2 strategy of marrying a lab. You simply have married you advisory board.
The debate inside these organizations after the first couple of years is usually the same:
Board member 1: Why are we always funding these same labs? This endogamy of having the advisors finance their own labs is disturbing.
Board member 2: Well, we chose as advisors the leaders in the field, so it is only natural that we also finance the best research… which happens to be the one coming from their labs.
Board member 1: I am not comfortable with the conflict of interest of having the advisors decide where the funding should go after seeing that so much ends up in their own labs or their friends’.
Board member 2: What should we do then? Remove the leaders of the field from our advisory board? Or stop funding the best research out there?
And then board member 1 leaves the organization.
My recommendation, if you allow your advisors to also receive funding, is to ask them to guide you through what should be the main research priorities but then use external reviewers to score the grant applications that fit those priorities. Ask the advisors to pick the priorities, not the awards. I would also consider having a condition to be part of the advisory board, which is that your lab cannot receive funding from that organization. For good established labs that are indeed the best in the field this should not be a problem (see comment on previous sections about bridge funding).
As a side warning: if you are a scientist considering applying to one of the call for grants of a patient group, make sure you review where their funding is usually going. You might think it is an open call for grants, while in reality it is a closed group of advisors and their closest collaborators getting as much funding as they can out of a patient organization. We all know examples of this.
Type 4: Owning the roadmap - the top-down approach
Anyone who has read the #ImpatientRevolution eBook knows that this is my preferred approach. Patient organizations cannot aspire to outfund NIH or their applicable national research funding body outside the US. What patient organizations can do with their research funds is to complement it, by identifying those areas that are essential for developing new medicines for their disease and that are currently not being pursued (or not fast enough). Then they would focus on those areas, ideally on setting up the basis so that good scientists can be attracted to those areas and bring more (non-patient) funding. This is how some patient groups are doing research.
I won´t extend on this strategy because the eBook covers much of it. But before wrapping up, here are some aspects of research funding strategy that I would like you to consider:
a) Bottom-up vs top-down
Do you want to collect external ideas and fund the best ones or do you want to analyze the field, identify the gaps and address those first?
Depending on your finances I recommend following a top-down strategy only, or a combination of both. For example:
Fund some projects that the organization has identified as a priority for the field. In those areas you identify what is needed and then go out and find the best lab or contract research organization to do that. This is probably the boring science, the type that no academic lab would propose in a call for grants because it is not conductive to exciting publications and promotions. It is, however, needed to develop cures.
At the same time, have your advisors identify where your priorities should be (for example biomarkers and a gene therapy with large capacity virus), and then have a call for grants on those topics. The call for grants would then be evaluated by a broader team, not just the advisors.
b) Projects vs enabling
I believe the greatest return on investment comes from generating the tools that will enable the scientists to then work in your disease and bring in their own external funding. For example, make sure that there is a good antibody, a good mouse model, iPSCs from patients, and that all of this open access. Make sure you have identified and helped set up clinical centers of excellence, and maybe some biomarker or outcome measure. Make sure there is a registry, or at the very list a way to reach out to families (a contact database). This toolbox serves both as a starter package and as a strategy to de-risk the field. With these things in place you will attract researchers and companies to your disease, and they will bring their own funding. They will also be many more than those that you could have directly funded yourself, so you would have mobilized much more funding to your disease than what you could have raised in decades.
Many of those efforts are the boring science, so you will only identify them if you do some top-down thinking and analysis. You should decide what percentage of your efforts and resources should be allocated to the boring science (enabling tools, de-risking the field), and what percentage will go to giving an opportunity to new exciting projects to develop.
c) Organization aging and cycles
Young organizations and old organizations think differently. The first two strategies I covered, for example, are much more common in younger organizations. I would recommend you to revise your strategy every 3 years and see what is working for you and what is not working. Older organizations also tend to grow more complex, and are more likely to engage professional staff.
I tend to work with patient organizations where the members of the organizations are parents of children with severe rare diseases. This has also helped me see another pattern, with parents of younger children being more optimistic and driven and investing in more high-risk projects, while parents of older children being more likely to make safer investments or to even move away from research and into care support programs. A parent of a 3-year old might want to invest in gene therapy, while the parent of a 13-year old might be more concerned with developing devices to assist with mobility issues, for example.
And add to this complexity the burnout factor. I´ve seen organizations that are centered around one person that can last many years, but those that rely on a group of core members can experience changes over time. I estimate a 3-5 year burnout time, before new members need to take over and continue the life or the organization.
Because of this, the same organization in year one is very different to the same organization in year seven. The members are different, the strategy is different, and what they mean when they say “we do research” is also different.
Let’s start an #ImpatientRevolution!
Ana Mingorance, PhD
Impatient series #0 – The impatient series
INTRODUCTION TO THE SERIES - The field of rare diseases, where each disease affects only hundreds or thousands of patients world-wide, is leading this revolution. Their diseases were once orphan to medicine, too rare to receive sufficient attention and investment to move the needle. Today patients take ownership of their research fields, and move the needle themselves. They have become the center of the network and the expert in the room. They are impatient, not wanting to wait for pharma to take an interest in their disease. They are impatient patients and are leading an impatient revolution.
The way that medicines are discovered starts in a lab, either at a university or at a pharmaceutical company, and when the discovery seems promising a pharma company picks it up and spends hundreds of millions of dollars to get it through clinical trials and into your nearest pharmacy shelf.
Until recently, patients only showed up in this process as volunteers for the clinical trials and as the final customer once the drug reached the market.
I’ve trained as a scientist, one of those that first discover things in the lab, but for the last 7 years I have been working with patient organizations. I have been able to witness (and join!) a true revolution in the way we discover medicines, with patients and patient groups taking a leading role from the very beginning. The field of rare diseases, where each disease affects only hundreds or thousands of patients world-wide, is leading this revolution. Their diseases were once orphan to medicine, too rare to receive sufficient attention and investment to move the needle. Today patients take ownership of their research fields, and move the needle themselves. They have become the center of the network and the expert in the room. They are impatient, not wanting to wait for pharma to take an interest in their disease. They are impatient patients and are leading an impatient revolution.
A keyword that has become mainstream in these recent years is “patient engagement”, with companies wanting to reach out to patient groups but not sure of what patient engagement would actually involve, what best practices apply, or how they can measure the value of those interactions. The industry is following the patients in this revolution, and learning from them.
I published a free eBook in February of 2017 called the #ImpatientRevolution that summarizes much of my learnings as a scientist working with rare disease patient organizations. It is written with the impatient patients in mind, and talks about how drug discovery works, what they can do to advance research from a patient organization, and how they can engage with the pharmaceutical industry. That´s right, there is much to say about “corporate engagement” as well.
Over the past two years I’ve found myself in many conversations with groups of patients and caregivers that were just starting an organization and wanted to know the best way to do that. The eBook has been a very useful reference in all of these cases, but there is more content and more topics that are not necessarily fully covered in #ImpatientRevolution.
To give me the flexibility to keep adding new content that will serve as a reference for many future conversations, I have decided to start a series of articles called the Impatient Series, and publish them here under Dracaena Report.
Some of the topics I want to include are:
What patient organizations mean when they say they are doing research (different formats that I’ve seen, when to choose one or another)
What patient organizations can do to advance preclinical research, even before the pharma industry gets interested in their disease.
How to make research funding decisions (different formats, when to choose one or another)
How to evaluate and track projects to make sure your money is well-spent.
What makes pharma companies choose one disease vs another one.
What are the types of drugs that could treat rare diseases and what research is needed for each of them.
Working with the pharma industry: NDAs, trust and more.
I also want to address a tricky topic that often comes up when a new patient group finds me and asks me for advice. Their questions are usually followed by “can we hire you to guide our research efforts?” and my answer is invariable no, I can give you some strategic advice but you need to find someone else (I´m only one person with 24 hours in each day). This is usually followed by “where can I find another scientist that could work with us?” and I don’t have an easy answer for that. We are not talking about your usual scientific advisor that remains comfortably seated in their academic chair, but about finding a scientist with the knowledge and entrepreneurial instinct that patient groups need and who is willing to roll up their sleeves and step inside the world of patient organizations.
But I think there is a solution for this because in the last 12 months I’ve started to also come across the reverse situation, scientists who approach me to ask if I think working for a patient group would be good for them and how to find a good patient group. So I am very excited to be adding some new topics to these series that were never covered in the original #ImpatientRevolution Book:
How do I find a scientist that could work with our patient organization.
For scientists: what to consider if you think you might want to work with a patient organization.
…and more related questions to come
As a final note, it goes without saying that this is all personal advice and insights based on my very own experience. I look at the evolving landscape of social entrepreneurship in the patient community, followed by adaptation in the pharma industry worried to be left behind, and try to understand the model that explains it and the rules that it follows. Your experience might be different, and I would love to hear from you. Overall I hope the series will become both a useful resource and a door to dialogue.
Let’s start an #ImpatientRevolution!
Ana Mingorance, PhD
IMPATIENT SERIES ARTICLES:
#1 – WHAT PATIENT ORGANIZATIONS MEAN WHEN THEY SAY THEY ARE DOING RESEARCH
#2 – WHAT ARE COMPANIES LOOKING FOR WHEN CHOOSING A RARE DISEASE
Main lessons from the European Congress on Epilepsy 2018
Every other year the International League Against Epilepsy organises a major epilepsy medical congress in Europe called the European Congress on Epilepsy (ECE). This year I attended the main three days of the ECE addition in Vienna, looking at the field partly as a drug developer and partly as a patient advocate working on behalf of rare epilepsy patient communities. Here is the list of what I found the most interesting at the ECE 2018 meeting.
Every other year the International League Against Epilepsy organises a major epilepsy medical congress in Europe called the European Congress on Epilepsy (ECE). This year I attended the main three days of the ECE addition in Vienna, looking at the field partly as a drug developer and partly as a patient advocate working on behalf of rare epilepsy patient communities.
Here is the list of what I found the most interesting at the ECE 2018 meeting:
1. The big players are gone, the orphan players are taking over.
I missed seeing the large stands in the exhibition hall that UCB or GSK would always have. Instead we are now surrounded by (often) smaller companies that are launching molecules to treat orphan forms of epilepsy. It is an evolution that we also see in other disease fields, and that started becoming clear in epilepsy a couple of years ago. As rare diseases become more popular for drug development and we have more approvals, not only we see more orphan drugs at epilepsy conventions but we also see different players.
If you turned around at the ECE 2018 meeting you could see the stands of GW Pharmaceuticals, fresh from their FDA approval for Epidiolex for treating Dravet and Lennox-Gastaut syndrome (expected EMA decision 1H 2019); Biomarin, which develops Brineura, with a stand focused on CLN2 (one of Batten disease types); Biocodex, with the newly FDA-approved stiripentol for treating Dravet syndrome (approved in EU since 2007); and Novartis with a stand on Tuberous Sclerosis Complex for their also recent approval of Votubia/Afinitor.
In addition to those, Zogenix, in very late stages of development of fenfluramine for the treatment of Dravet syndrome, didn’t have a stand but sponsored a symposium on Dravet and had multiple posters. I would expect to see Zogenix and potentially Ovid Therapeutics get some more visibility at the next American Epilepsy Society meeting given their active late-stage programs in Dravet and Lennox-Gastaut syndromes. But for now, at the European meeting, we could already see four orphan epilepsy syndromes replacing much of the exhibition floor space formerly dedicated to medicines with a much broader spectrum. Since I focus on rare epilepsy syndromes, seeing that many drugs coming to the market and so much research on rare syndromes with epilepsy makes me very happy.
2. Epileptologists discussing much more than just epilepsy.
Probably as a combination of paying more attention to rare epilepsies, which often have many serious and disabling comorbidities, and to patient-centricity and patient-empowerment becoming more mainstream, the epilepsy specialists start talking about these epilepsies in a much boarder way which captures much better the patient and caregiver experience. While years ago most presentations would focus on seizures, at the ECE 2018 we also talked about how to explain families a new diagnosis, the impact on caregivers of having a child with a rare epilepsy, the impact on siblings as well, and many aspects related to intellectual disability or psychiatric comorbidities. This large conversation shift was very refreshing for those coming from the patient side, and is likely to lead to much better patient outcomes.
3. A lot of exciting new therapies are coming, in particular for genetic forms of epilepsy.
Many presentations on the future of epilepsy treatments highlighted the new trials that are coming, most focused on orphan forms of epilepsy. In addition to Epidiolex and fenfluramine (for Dravet and Lennox-Gastaut syndromes) several presentations highlighted ganaxolone from Marinus for treating CDKL5 deficiency disorder, XEN1101 from Xenon for KCNQ2 encephalopathy, and OV935/TAK935 from Ovid Therapeutics and Takeda for multiple rare epilepsies as well.
There are also hopes about future genetic therapies able to increase expression from the heathy gene copy, in all of those cases where the disease is caused by a de novomutation in one of the gene copies. In fact, I had the pleasure to meet with one executive of Stoke Therapeutics attending the conference and hear about their program to use an antisense treatment in Dravet syndrome that will increase the missing protein expression. They are still at a preclinical stage, but from my conversation I came out of the meeting convinced that they are doing an excellent work and that they are well-prepared to take into clinical trials, in some years, what could be the first disease-modifying treatment for Dravet syndrome.
I missed hearing a bit more about gene therapy approaches, which we know are also in development for several of these rare epilepsies where the genetic problem is due to a loss-of-function or a loss-of-expression of a gene. I´m sure it won't be long before these therapies are discussed at epilepsy meetings alongside more classical pharmacological approaches.
4. It is never too late to improve the life of a patient living with a severe epilepsy.
As we talk about those potentially disease-modifying treatments, and at the very least disease-targeting treatments, one hope and one fear emerge. The hope is that they will help us treat not only the epilepsy but also the cognitive, psychiatric, motor and other problems that the patient experiences, since they will target the root of the disease. The fear is that beyond certain age, they might do little for the patient.
I have to thank Prof Sanjay Sisodiya from UCL who works with adults with difficult-to-treat epilepsies for his beautiful and inspiring presentation on diagnosing genetic epilepsies in adult patients. He showed us the image of the medical records of a patient with drug-refractory epilepsy and intellectual disability in his late 50s that was the size of an encyclopaedia collection. Going through that much medical history to try to diagnose the patient would represent an enormous challenge to any physician. The patient’s disease was so severe that he had even stopped talking, so one might think that knowing the specific cause wouldn’t make much of a difference to this patient condition. It turns out the patient had Dravet syndrome, which could be identified in a genetic test, and this finding enabled his doctors to remove the contraindicated medication that he was taking and prescribe instead the standard of care for this particular syndrome. The patient condition improved to the point that Prof Sisodiya showed us a video of the patient, now 66, having a conversation with him.
“It is never too late” was the message to the audience.
It is never too late to diagnose a condition, and it is never too late to offer the patient the best medication that is currently available for their disease. This will be particularly important once we have actual disease-targeting therapies in the market, and only then will we be able to know how much improvement is achieved at each age.
SUMMARY
Overall, an evolution of epilepsy therapy discovery from treating symptoms to treating causes is clear. Much of this is driven by improved understanding of the genetic causes of many types of epilepsy, which points to potential new targets for therapies, and much is driven by the progresses in technologies such as the development of more specific ion channel modulators or antisense strategies that now enable us to tackle those targets.
The second major evolution, linked to the previous one, is a greater focus on rare (orphan) epilepsies over the large symptomatic market. This evolution, as is other disease fields, follows a combination of business advantages and better therapeutic fit, given that many of the rare epilepsies have a genetic cause. For example, as one of the most common genetic forms of epilepsy, Dravet syndrome is likely to be the first --or one of the first-- syndromes to get a disease-modifying treatment using these new approaches.
And last, I have seen another evolution in the way that epileptologists talk about the epilepsies at their major conferences, which has become more holistic and patient-centric. When I started working in the epilepsy therapeutics field 9 years ago, the view of epilepsy by the epilepsy specialists and the pharmaceutical companies was much more focused on the seizures and less on the overall patient experience. Now, as a result of much patient advocacy, physicians look at the disease the same way the patient and their families do. Companies and regulators have also changed the way they used to operate, now including much more directly the patient perspective as part of their work, which has expanded the way they see the disease and think about treatments. And in a medical conference like the ECE 2018, this change was very visible.
I personally missed hearing more about CDKL5 deficiency disorder and other “less popular” rare epilepsies, and about some of the non-pharmacological therapeutics in development, such as antisense therapies and gene therapy approaches. Next stop for the epilepsy community is the American Epilepsy Society meeting starting November 30thin New Orleans where I hope to hear more about those topics, and report back.
Ana Mingorance, PhD
En Español - Fármacos en camino para el síndrome de Dravet
Dravet syndrome pipeline review 2018 - Summary in Spanish for Dravet families.
Los avances en torno al tratamiento del síndrome de Dravet en los últimos años han seguido pasos agigantados. En los últimos 5 años hemos experimentado una explosión en el número de programas en desarrollo para tratar la enfermedad: de tener solo Diacomit y nada más a tener Diacomit y 14 más en desarrollo. Este texto es un resumen en Español del Dravet syndrome pipeline review 2018 dirigido a familias, con el texto no solo traducido sino también ajustado a los intereses de aquellos que tienen un hijo/a con síndrome de Dravet y su entorno.
¿Sabíais que hay 14 terapias en desarrollo para tratar el síndrome de Dravet? ¿y que varias de las terapias no buscan solo controlar las crisis epilépticas sino corregir el defecto genético que causa la enfermedad?
Los avances en torno al tratamiento del síndrome de Dravet en los últimos años han seguido pasos agigantados. En los últimos 5 años hemos experimentado una explosión en el número de programas en desarrollo para tratar la enfermedad: de tener solo Diacomit y nada más a tener Diacomit y 14 más en desarrollo.
Por segundo año publicamos una revisión de los fármacos en desarrollo para tratar el síndrome de Dravet (ver entrada).
El texto original va dirigido a empresas interesadas en el sector o que ya están desarrollando fármacos, así como otros profesionales relacionados con la industria farmacéutica. Este año hemos querido ofrecer también un resumen en Español dirigido a familias, con el texto no solo traducido sino también ajustado a los intereses de aquellos que tienen un hijo/a con síndrome de Dravet y su entorno. Hemos incluido texto y una figura adicional dirigido a esta audiencia, y eliminado secciones que no son tan relevantes.
El texto describe todos los fármacos en desarrollo para tratar el síndrome de Dravet a fecha de julio de 2018 y plantea las direcciones futuras.
Esta adaptación de cómo están todos los fármacos en desarrollo para el síndrome de Dravet la dedicamos a los guerreros de la Fundación Síndrome de Dravet, que han contribuido de manera directa a muchos de los logros que resumimos en la revisión.
Un sueño... una meta.
DRAVET SYNDROME DRUG DEVELOPMENT PIPELINE REVIEW 2018
The 2018 Dravet Syndrome Pipeline and Opportunities Review provides a review and analysis of 14 drug candidates in development for the treatment of Dravet syndrome, including 9 products that have received orphan drug designations. It also includes an analysis of the competitive landscape and evaluates current and future opportunities of the Dravet syndrome market.
It has been a year since we released the 2017 Dravet Syndrome Pipeline and Opportunities Review, a market research publication that provides an overview of the global therapeutic landscape of Dravet syndrome.
There have been many developments in the last year, including the approval by the FDA of Epidiolex (cannabidiol) by GW Pharmaceuticals for the treatment of Dravet syndrome, the announcement of positive data from two Phase III clinical trials with ZX008 from Zogenix, and the arrival of a new company pursuing a disease-modifying antisense-based approach (Stoke Therapeutics).
The 2018 Dravet Syndrome Pipeline and Opportunities Review provides a review and analysis of 14 drug candidates in development for the treatment of Dravet syndrome, including 9 products that have received orphan drug designations.
The report includes the most recent updates on Epidiolex (cannabidiol) from GW Pharmaceuticals; ZX008 (fenfluramine) from Zogenix; Translarna (ataluren) from PTC Therapeutics; OV935 (TAK-935) from Ovid Therapeutics and Takeda; EPX-100, EPX-200 and EPX-300 from Epygenix Therapeutics; ZYN002 (transdermal cannabidiol) from Zynerba Pharmaceuticals, BIS-001 (huperzine) from Biscayne Neurotherapeutics; PRAX-330 from Praxis Precision Medicines; SAGE-324 from Sage Therapeutics; OPK88001 from OPKO Health; XEN901 from Xenon Pharmaceuticals; and an ASO program from Stoke Therapeutics.
The 2018 Dravet Syndrome Pipeline and Opportunities Review also includes an analysis of the competitive landscape and evaluates current and future opportunities of the Dravet syndrome market.
The report is now available in this site.
Ana Mingorance PhD
What you should know about patient data after May 2018
After May 2018 patients will have the right to request access to their data files. They will have the right to withdraw their data from a database that is not being used in the way that the patient through it would operate. And they will have the right to obtain these files generated by a particular data holder, for example a gene testing lab, and get the usable file and share it with as many studies and registries as the patient wants. The EU General Data Protection Regulation gives patients the Right to Access, the Right to be Forgotten, and the Data Portability right.
When scientists don’t fight over funding they fight over data.
Having large amounts of data that competitors can’t access means having the upper hand when it comes to publishing and getting funding. Corporations operate the same way. Science is, after all, a knowledge economy, where data is knowledge and knowledge is power.
But in a world where data is seen as an asset, patient rights when it comes to accessing their own data and deciding what to do with it can be compromised. And they are, in a regular basis.
I am a vocal advocate for empowering patients (and individuals in general) by giving them access to their personal data files so that they can know what these contain and choose whom to share it with. I also know that not everybody feels the same way. I have had the opportunity to address data scientists twice, first in 2015 and more recently at a personalized medicine meeting in 2017, and both times I managed to divide the audience and offend some data scientists and clinicians.
The good news is that this debate is becoming obsolete in May 25th 2018, when a new regulation protecting patients right to access their data will enter in force.
The surprising news is that the solution didn’t come from a medical organization but from the EU Parliament, in the form of a General Data Protection Regulation. The key word here is “general”, because the new regulation was not written with patients or even with medical data in mind. It applies to anyone who collects other people’s data. From Google storing your preferences, to Facebook knowing your friends, to your aunt having a mailing list for her cooking blog. And patients are, surprisingly, a key collateral beneficiary of this regulation.
Let me review the current problems that patients face to access their own data and why the General Data Protection Regulation is so important.
CURRENT PROBLEMS AROUND PATIENT DATA
Before 1973 your doctor didn’t need to tell you what he thought was your diagnose, or to ask you to consent to the treatment he was prescribing. This would be unthinkable in today’s world, but it was grounded on the observation that the doctor, and not the patient, is the most knowledgeable person in the room when it comes to medicine. While this is generally true, it is also paternalistic and condescending, and in 1973 the American Hospital Association adopted the Patient’s Bill of Rights to put an end to this practice. After the Bill, patients were entitled to receive information about their disease and to make decisions about treatments (except in emergencies), among other important rights. Similar regulations were then adopted worldwide.
When I give presentations on this topic I like to use the slide below to illustrate the problem of scientists and healthcare providers not supporting the transfer of patient data from a study to another. It shows the example of a patient having to repeatedly give the same personal and medical information over and over to their hospital, a patient registry for their disease, to a couple of academic studies, and probably also to a clinical trial where the patient participates and that is also generating a separate patient registry. The burden on that individual patient is ridiculous, and this is not a made up example. I know multiple cases of patients with rare diseases where they end up interacting with such a large number of data holders in an attempt to get the best medical care and to help advance the scientific understanding of their disease.
There are two separate problems here:
1- Data holders refuse to share the patient data with other data holders.
The main argument is often that the informed consent didn’t consider sharing data with third parties, but in my experience even if the patient (or caregiver) offers to sign a new informed consent to support that transfer the original data holder refuses. As I see it they are not thinking on the patient’s best interest but looking at the data instead as an asset that they own.
2- They also often refuse to share the patient data with the patient himself.
If patients had access to their entire medical history and relevant studies in a way that can be easily transferred to other data holders, such as new registries and studies, the patient would choose who has it and who doesn’t. But they don’t have that choice when they don’t have their data files. The expressed reasons to not grant the patient access to his or her own data files fall often into two categories. One is the technical one, like in the real example: “we never designed our registry with a output format that could be useful to you”. The second is the same one that led to the 1973 Patient’s Bill of Rights, as in another real example: “no we can’t give you your whole exome sequence because you don’t understand genetics, interpreting sequences is hard and confusing, and you might make the wrong decisions based on uninterpretable data”.
The cry of patients for accessing their own genetic sequence data files is so loud that in 2015 the European Society of Human Genetics issued a press release with the following headline: “People want access to their own genomic data, even when uninterpretable”.
It wasn’t about patients having the knowledge to read a raw whole exome sequence file, but about being entitled to get a copy of such file.
I personally thought it would have to be an organization like the European Society of Human Genetics or the American Hospital Association who would issue a new recommendation (or hopefully something more enforceable) to protect patients right to access their own medical data files. Surprisingly the regulation that would come to protect these patients rights was simply a new general regulation for personal data protection that was never developed thinking specifically of patients.
WHAT THE GDPR ACHIEVES
The General Data Protection Regulation (GDPR) was approved by the EU Parliament in 2016 and will enter in force in May this year. It doesn’t matter where the organization that collects personal data of data subjects (including medical data) is located. What matters is that if the data subject resides in the EU, the organization needs to be compliant or face heavy fines. Unless organizations are planning to follow separate Standard Operating Procedures for EU and non-EU residents, most will simply have to be compliant with the GDPR and the new regulation will also protect non-EU patients.
My favorite “data subject rights” are the following:
1- Right to Access.
Under the GDPR the data holder will have to provide patients with a copy of their personal data, free of charge and in a machine-readable format. It won’t be ok to argue that the database wasn’t built for export functions or to be passive-aggressive and give the patient only a small summary in a PDF. The patients, and all data subjects in general, are now empowered to have a copy of their data files, to know how much data the data holder has collected from them, and to know what they are using it for.
2- Right to be Forgotten.
Under the GDPR the data holder can withdraw consent and request their data to be removed from the data holder files and to not be user or disseminated. If you are a patient that gave data to a registry on the understanding that they would use it to further research in your disease, and you later become aware that they are refusing to share data with other research groups, you can now approach the registry and request your data to be removed.
3- Data Portability.
Under the GDPR the patient can request the data holder to provide them with a copy of their files in a machine-readable and usable format not only for their own records, but also to transfer them to another data holder. In my real example of a rare disease patient that has his data in multiple registries and studies, the patient can now get usable files from his hospital including proper gene sequencing files and then share this file with the other registries and studies. They won’t have to give the same data, and be subject to the same procedures, over and over as it happens nowadays.
AFTER MAY 2018
After May 2018 I will have to change my PowerPoint presentations. Patients will have the right to request access to their data files. They will have the right to withdraw their data from a database that is not being used in the way that the patient through it would operate. And the patient has the right to obtain these files generated by a particular data holder, for example a gene testing lab, and get the usable file and share it with as many studies and registries as the patient wants.
In 2015 I offended some data scientists at a big data meeting with the following message:
“Health data must be shared
In particular with the patient (even if they don’t understand it)
Because it is their data
And because they will share it”
I wanted to shift the conversation on giving patients’ access to their own data from “the patients don’t understand the data” to “they need to have access so that they are able to share their data”. I guess we don’t need to debate this anymore. After May 2018 we just need to say that it is the new law!
Ana Mingorance PhD
Big gene, small virus
There is no doubt that AAVs will support the development of many gene therapies for genetic diseases, but for many diseases AAVs are too small to carry a copy of the gene that patients need. There is a strong need to find non-AAV alternatives that can provide a suitable gene therapy option for those diseases caused by mutations in large genes. Within Dravet syndrome, these next-generation therapies are being led not by companies but by academic groups with the support of patient organizations. This is a review of these programs and how they are attempting to develop a gene therapy for treating Dravet syndrome.
We wrapped up 2017 with the news of the first gene therapy approved in the US to treat a genetic disease. It was Luxturna, approved for treating vision loss in patients carrying mutations in a particular gene that causes retinal dystrophy. The videos of vision improvement in patients treated with Luxturna that Spark Therapeutics has shown are breathtaking, and I trust gene therapy will one day be the go-to treatment for most genetic diseases.
There are in fact many genetic diseases! From the estimated 6000 to 8000 rare diseases, the large majority is thought to be also caused by gene mutations. And the most common consequence of those mutations is loss of function of the gene, so the approach that Luxturna used to deliver a backup healthy copy of the gene to the affected cells would be the ideal one.
But here is the catch: Luxturna uses a small virus, called Adeno-Associated Virus or AAV, to deliver the therapeutic gene to the affected cells. AAV is the gold-standard when it comes to gene therapies in development, with many advantages over other virus that are less infective (don’t get into as many cells) or might trigger an immune response. But AAV can only transport small genes, and not all diseases have mutations in small genes.
In a recent Dravet syndrome families meeting, a gene therapy expert explained to the parents that that scientists have been very good at developing cars able to transport a healthy gene copy to the patient, but that their particular disease will need a bus. I like the metaphor very much.
Many diseases will need virus larger than AAV to be able to have a gene therapy. We need a bus.
CAN WE MAKE GENES SHORTER?
In some diseases that have “big genes” it might be possible to develop shorter versions of the gene that could fit into an AAV and retain sufficient functionality to have therapeutic benefit. The main example that comes to mind is the mini-dystrophin gene, which is currently in clinical trials (here and here). This approach is only possible because dystrophin is a large scaffold protein that needs to attach to multiple partners and can be reduced to only those main protein domains retaining functionality.
Dravet syndrome is caused by mutations in the SCN1A gene, which produces an ion channel that needs to cross the plasma membrane 24 times. Such a large protein needs to go in an out the membrane the right number of times and to have the right loops inside and outside to be able to form the correct channel structure and functionality. There is no spare protein bit that we could chop out of the gene to reduce it to a size where it could fit into an AAV. We need a bus.
LOOKING FOR A BUS
Dravet syndrome is not the only disease caused by mutations in a gene too large to exploit the great advances in using AAV as therapeutic vehicles for gene therapy. The entire field of gene therapy needs to find the best solution for the next-generation therapies that go beyond what AAV can give us.
Within Dravet syndrome, these next-generation therapies are being led not by companies but by academic groups with the support of patient organizations:
|| Dravet Canada and the US Dravet Syndrome Foundation are supporting a gene therapy project at Toronto University that will use AAV to deliver compensatory genes (not SCN1A) for the treatment of Dravet syndrome. Because the AAV technology is the most advanced one this approach offers the fastest gene therapy path to the clinic, but it doesn’t represent the ultimate ideal treatment that would involve the delivery of a healthy copy of the SCN1A gene to the affected neurons.
|| One of the projects aiming to develop that ideal treatment is led by Simon Waddington and Rajvinder Karda from UCL. This team is working on one type of “bus”, Lentivirus, that has enough capacity to carry the SCN1A gene and become a treatment for Dravet syndrome. They have received the support from Dravet Syndrome UK and share updates with other interested patient groups.
|| And a larger collaboration was recently awarded a European grant to also look for a different type of bus. This time scientists from Spain, France and Israel are trying to develop a gene therapy for Dravet syndrome using Adenovirus, another type of high-capacity virus. They are collaborating with Dravet Syndrome Foundation Spain and the Dravet Syndrome European Federation, and have also developed a close relationship with patient groups.
If everything goes well, one or more of these programs will "cure" a mouse with Dravet syndrome using one of these virus within the next 2 to 3 years. The next step are clinical trials. This all used to look like a very distant future, but with the approval of Luxturna it has now become a reality. Soon someone will get a gene therapy using a big virus approved, and that day Dravet and many other syndromes caused by mutated large genes will have found a bus.
Ana Mingorance PhD