Epilepsy Insights
MAIN LESSONS FROM THE 2025 CDKL5 FORUM
For the past eleven years the Loulou Foundation hosts an annual meeting where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances.
Here are the main news and take-home messages from the 2025 CDKL5 Forum that took place in October 27-28 in Boston
For the past eleven years, the Loulou Foundation has hosted an annual meeting, the CDKL5 Forum, where scientists and drug developers working on CDKL5 Deficiency Disorder (CDD), together with clinicians and representatives from patient organizations, meet to discuss the latest developments in the field and to advance towards treatments and cures. You can find summaries from some of the last meetings here: 2018, 2019, 2020, 2021, 2022, 2023 and 2024.
The 2025 CDKL5 Forum edition took place October 27-28 in Boston, and will return to London next year for the 2026 edition. This was again the largest Forum to-date, with over 200 participants including representatives from 27 companies and 22 national patient groups.
I will try to summarize the main take-home messages from this year’s Forum. This won’t cover all the presentations, so I apologize in advance to all the scientists and doctors whose work I won’t cover. My focus will be on the main themes that we saw and the main progresses towards treatments.
1. Expanding our (useful) knowledge of CDKL5
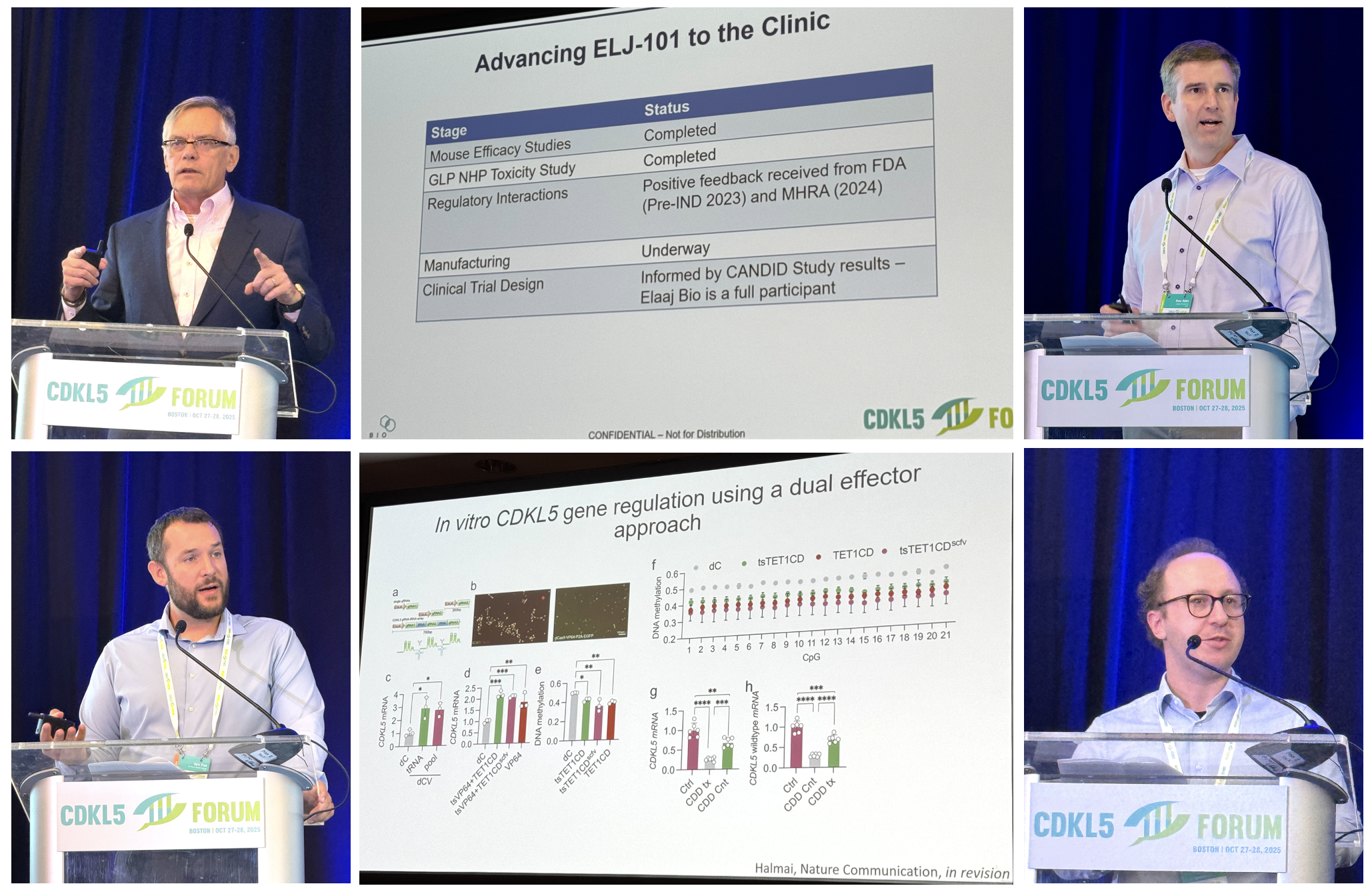
Image: Forum Director Dr Dan Lavery (Loulou Foundation); Dr Enya Paschen (Ulysses Neuroscience); Prof Peter Kind (University of Edinburgh); summary of some CDKL5 direct targets; Ulysses Neuroscience seizure platform.
Before this 2025 Forum, I thought that maybe we have uncovered most of what we need to know about how the CDKL5 kinase works, and therefore any new discovery would barely move the needle. And boy was I wrong! At this Forum, we learnt about multiple discoveries for how CDKL5 plays roles far beyond what we thought. And many of these, are useful for treatment design.
For example, we learnt about a discovery made at the same time in two countries that CDKL5 controls a protein that binds to RNA, which means that when CDKL5 is active it not only turns on and off other proteins (as kinases do), but it also controls how much of other proteins gets made. CDKL5 is also involved in autophagy, which is how cells get rid of protein aggregates. So that is another way to control how much or how little of other proteins stays in the cell. And another lab discovered how CDKL5 controls several synaptic proteins because it has a Velcro-like domain that sticks to synaptic proteins (a PDZ-binding domain). And these new functions are added to all what we knew already! like CDKL5’s control of the ion channel Cav2.3, or its modulation of microtubule dynamics and plasticity.
What this means for scientists like me who work in therapy development is an expanding map with more new targets, and therefore more therapeutic directions that we can try to pursue. So it might look like more complexity is bad, but it is actually good news. We also had a presentation from Ulysses Neurosciences showing their mouse testing platform for CDD, including behavioral readouts in male and female mice and also seizures and EEG readouts, which will make it easier to evaluate all those therapeutic options.
Bottomline: scientists are still mapping the “dark matter” of CDKL5 biology, and each of these new discoveries is a potential clue into how to develop therapies for CDD. The 2025 Forum was exceptionally fruitful on this front, with many new biology discoveries.
2. More options to access new medicines than ever before
Image: CDD pipeline listing most programs in development; moderator Prof Orrin Devinsky (NYU Langone); Angel Neurotherapeutics program; fenfluramine activity (UCB Pharma); Bexicaserin trial recruitment sites (Lundbeck); Praxis CSO Prof Steve Petrou.
During his introductory remarks, Dr Dan Lavery from the Loulou Foundation showed a slide of the CDD pipeline, showing multiple generations of small molecule drugs and gene therapies all in trials or advancing towards trials for CDD.
And the most important thing: in 2025 we have one drug approved, a second one having successfully completed Phase 3 trials, and two global Phase 3 trials actively recruiting patients with CDD. There was never a year before like this 2025.
This is the first year that we had Immedica at the Forum, after their acquisition of Marinus. The Immedica team presented their learnings about a slower titration with ganaxolone to reduce side effects. Ganaxolone is already commercialized in the US, and Immedica is working on market access for other countries.
This summer, UCB Pharma announced that their Phase 3 trial with fenfluramine in CDD had been positive, and at the Forum they shared the complex mechanism of action of fenfluramine, which includes more than the serotonin receptor 5HT2C. They will announce the exact results from the CDD trial at the American Epilepsy Society meeting in December, so we will watch for those. UCB will file for expanding the label of fenfluramine to also include treatment of seizures in CDD.
Last year, the regulatory agencies started allowing companies to run clinical trials combining different epilepsy syndromes and not just one at a time. This has resulted in new clinical trial options for CDD. And because these trials ask for only 4 countable seizures a month and include children as well as adults, they have given us more options than ever to try new epilepsy medicines as part of clinical trials. At the Forum we saw two large Phase 3 trials that are currently recruiting across many countries:
Lundbeck is running the DEEp OCEAN Phase 3 trial with their drug bexicaserin. Bexicaserin uses one of the receptors for fenfluramine, 5HT2C, and had very good efficacy in the Phase 2 trial also in a mix of syndromes. They have about 100 trial sites in the US, Australia, Europe and Asia. Here is the link to the study: https://deepdeestudy.com/
Praxis is running the EMERALD Phase 3 trial with their drug relutrigine. Relutrigine is a next-generation sodium channel blocker with many convenient attributes: no titration needed, once a day dosing, liquid formulation, and the trial can be done from home – they send a doctor and a nurse to your place. The trial is also in many countries, and the website is in 12 languages. Here is the link to the study: https://www.resiliencestudies.com/emerald
In addition to this first generation of treatments, all design to reduce seizure frequency, we got a glimpse for what we might have in a few years: the precision medicines for CDD. These are drugs that can correct some of the key aspects of the CDD biology. This year, we got a presentation from Dr Massimiliano Bianchi from Angel Neurotherapeutics showing their preclinical work with a drug called PME and related analogues. These drugs bind to cytoskeleton-binding proteins to compensate for the loss of CDKL5, and the most advanced drug could reach trials in CDD by 2027. This is one of multiple shots on goal to have precision medicines for CDD reach clinical trials in about two years, and because they target key aspects of the CDD biology, they are expected to also improve non-seizure symptoms as shown for the Angel lead program.
Bottomline: CDD families currently have more access to new experimental therapies than ever before. And this also includes adult patients that had not been eligible for trials until now.
3. Gene therapy progress, and a lesson in safety
Image: Keynote speaker and moderator Prof Jim Wilson (Gemma Bio); Elaaj gene therapy program (Loulou Foundation); Dr Russ Addis (Loulou Foundation); Dr Kyle Fink (UC Davis); UC Davis X reactivation project; Andrew Steinsapir (Apertura Gene Therapy)
Gene therapy remains one of the major topics at the CDKL5 Forum, and this year we received messages of excitement and also caution. Some of the Alliance members at the conference told me how “it felt like real talk”, and how that made them feel that progress in gene therapies for CDD was more tangible and real than ever before.
We learnt about progress in THREE gene therapies for CDD that are in development:
1. Dr Sharyl Fyffe-Maricich from Ultragenyx spoke about their gene therapy program, addressing “the elephant in the room” which is that they can’t give us any timelines yet for when this program might progress into trials. But she gave us a valuable scientific gift, sharing with us their research in monkeys that shows that neurons that already make CDKL5 have room to make some more CDKL5 from the gene therapy. This is important for the many CDD patients with missense mutations, who make CDKL5 but it is not functional because it carries one wrong aminoacid. Sharyl’s research indicates that they are likely to end up having a mix of CDKL5 proteins, some coming from their mutated gene and some from the gene therapy, so this is very positive. I was very relieved and grateful to learn about Ultragenyx’s findings.
2. Dr Russ Addis from the Loulou Foundation presented an update on the gene therapy that the Foundation is advancing. This gene therapy has been made in collaboration with Prof Jim Wilson, using a virus very similar to AAV9 that is now being used in 4 other clinical trials. Inside, the virus carries a copy of the CDKL5 gene and it is administered by injection into the brain CSF. Russ showed us the dose-finding studies in mice with CDD to identify the best clinical dose, and told us that the toxicology studies in monkeys have already been completed. The Loulou Foundation is now starting manufacturing, which is the large production of the gene therapy so that it can go to trials, and is already designing the clinical trial. Russ explained that manufacturing will take “the better part of a year” so we are looking into a clinical trial authorization around the end of 2026 to start trials in 2027. The Loulou Foundation announced a partnership with Gemma Bio, Jim Wilson’s company, to share data from the other gene therapies that use this same design and that are now in clinical trials. And to advance this and other types of gene therapy programs, the Loulou Foundation has created a subsidiary called Elaaj Bio, that as Russ said has a “vision for families and patients have more than one option, and to have more chances to access these therapeutics”.
3. Dr Kyle Fink from UC Davis and his lab are developing a very cool gene therapy for CDD: the X reactivation therapy. It works by using a virus to deliver to each neuron the instructions to read the second CDKL5 gene in the second X chromosome that all girls have. These instructions are a type of CRISPR. He reminded us of how nice his gene therapy works in CDD mice, and all their new progress to turn this mouse science into a human medicine. The new progress included figuring out the best doses to use, and checking that it can work in human neurons (using patient-derived organoids). They have started manufacturing and will soon start the toxicology studies in monkeys to progress into trials. This version of the gene therapy uses a combination of two virus that are administered into the CSF, but Julian Halmai (another UC Davis Professor) is working with Kyle to make a miniature CRISPR version that will enable in the future to do this with only one virus.
We also learnt about one gene therapy program that has been stopped:
This was an interesting surprise. Ashton Brennecke from Biogen explained how Biogen had been working in secret on a gene therapy for CDD, and now that the company has terminated the program they wanted to share some of their learnings with the scientific community. In their case, their gene therapy was very similar to the ones from Ultragenyx and the Loulou Foundation, and they studied how to use EEG in mice as a measure for efficacy. It was very interesting to hear about how they optimized EEG methods to be able to see the gene therapy efficacy, but it was also sad to hear that Biogen has joined Amicus and PTC Therapeutics as companies that had started gene therapies for CDD but stopped them before getting to trials. This type of attrition is common in therapy development, and the reason why we need so many shots on goal.
And importantly, we heard about what the field has learnt since the first gene therapy was tried in the first patient in the 90s:
Prof Jim Wilson is of the pioneers in gene therapy development, and delivered a spectacular presentation about the history and learnings on this field. Jim is the former Director of the Orphan Disease Center at UPenn and current CEO of Gemma Bio, the gene therapy company working with the Loulou Foundation on the CDD gene therapy. He explained how they discovered early on that to get gene therapies we would have to fight the immune system, even if it took them 17 years to understand exactly some of the key challenges. He discovered AAV9, and has now put several therapies in clinical trials both using AAV9 and his new generations of virus. Jim dedicated the last part of this talk to address a key topic right now in rare diseases: the recent cases of patients death in clinical trials with intravenous administration of high doses or AAV. His lab has studied the toxicity of gene therapies in almost 500 monkeys, and Jim asked for transparency in the field so that when a company has some unfortunate patient death in a trial, they share the data for how it happened exactly. Only that way, Jim and others can develop preclinical (animal) tests to anticipate that safety issue before it happens in a trial.
Andrew Steinsapir, from the company Apertura Gene Therapy, talked about the capsid (virus) that they are developing for neurological diseases and that is delivered by intravenous administration. The virus is engineered to cross from blood to brain by binding to receptors in the brain blood system. There are previous cases of therapies that used large proteins (although not yet virus) that can also cross from blood to brain using that same receptor, so the Apertura team believes that they should not find unexpected problems once their first programs reach clinical trials. If proven safe in trials, this type of virus could be used to create a second-generation of gene therapies for CDD that instead of being administered by a surgeon into the CSF could be simply injected intravenously.
Last, Dr Basel Assaf, an excellent toxicologist working at Attentive Science, seconded Jim Wilson’s message that so far, the field has been able to use monkeys (non-human primates) to anticipate any toxicity problem that can be later found in patients. However, this has not been perfect, and there was recently a patient death in a clinical trial in another neurological rare disease using an intravenous gene therapy that was not predicted in the monkey studies. Basel also had a great way to help us understand what we mean by “high doses”: an intravenous gene therapy can use seven quadrillion viruses, which is more than the number of cells in our body!
As a reminder: the gene therapies that Ultragenyx, the Loulou Foundation and UC Davis are developing, are NOT delivered using high dose intravenous administration. They use smaller doses, and are delivered straight to the CSF in the brain.
Bottomline: The gene therapy field is learning about safety risks, which requires transparency and collaboration. We have multiple shots on goal to have a gene therapy for CDD, with the possibility for a first trial in 2027. My overall message from this gene therapy session was quite positive. As a CDD dad put it in social media, “real change is coming”.
4. We are probably ready to run complex clinical trials
Image: Panel discussion “Where do we stand today with clinical outcome measures for CDD?”. Dr Xavier Liogier (Loulou Foundation); Dr Barry Ticho (Stoke Therapeutics); Dr Heather Olson (BCH); Dr Jenny Downs (Kids Research Institute, Perth); missing in the image Prof Tim Benke (University of Colorado); Dr Billy Dunn (Loulou Foundation, former FDA)
For the past 5 years of so, we have been hearing about developing and validating outcome measures for running clinical trials in CDD that measure symptoms beyond seizures. Looking at the 2025 Forum presentations, it looks like 80% of the work has been done, and if we had to start a clinical trial tomorrow with a gene therapy we would know what measures to include.
Dr Xavier Liogier from the Loulou Foundation presented the latest update on the international CANDID study, validating for CDD scales that have been used to measure cognition, behavior and motor skills in other diseases, and that regulators accept for drug approvals. By December of this year, CANDID will have 100 patients monitored over 2 years with this collection of scales, giving us longitudinal data for how m much change is expected over a 2-year period. Several scales and subscales are suitable for CDD, and a manuscript is under review for publication. One learning from CANDID with important trial implications is that patients with CDD have one to five seizures a day, so most will qualify for the current seizure clinical trials. This is much more than other epilepsy syndromes.
Prof Tim Benke from University of Colorado, and Dr Jenny Downs from the Kids Research Institute in Perth, presented their work to develop novel clinical scales specifically tailored for people with CDD. The clinical study that Tim leads is also close to having 100 patients reach the 2-year follow up, and importantly it also includes EEG studies so it might help identify a biomarker to see what is happening inside the brain with a gene therapy. Meanwhile Jenny is developing a novel scale for communication in CDD, which is often the top priority for families, and was praised by a former FDA Director for the very hard and diligent technical work that they are doing in developing this scale.
At a panel afterwards, presenters, including ex-FDA Director Dr Billy Dunn and Stoke Therapeutics CMO Dr Barry Ticho, highlighted that clinical trials and patient care have different goals. While patient care requires you to look at the entire complexity of the disorder, and to focus on what matters the most to every single individual patient, it is “not necessary to show everything under the sun [in trials] to get one drug approved”. Billy Dunn warned about “creating unintended obstacles” for clinical trials, and advised developers that “you don’t have to put everything in the trial and put it all in the label”. Instead, he recommended using post-approval studies to get a broader picture of the drug potential, and let doctors explore the value for each individual patient once the drug is approved.
Bottomline: There has been much progress to develop and validate clinical scales for trials in CDD beyond seizures. Because of this work, if we had to start a clinical trial tomorrow with a gene therapy we would know what measures to include. This has been possible because of hundreds of CDD families that have been participating for 2 years (some even more!) in these observational studies where they don’t get any direct benefit, only burden. You should all be proud for making this happen!
5. A strong global community and a global alliance of hope
Image: Lili Hass message and picture (CURE5); Dr Maria Luisa Tutino message; Dr Maria Luisa Tutino together with Lynn and Majid Jafar; Dr Katheryn Frame presentation and picture (CDKL5 Alliance)
The international community, and in particular the CDKL5 Patient Alliance, were repeatedly described as “a global alliance of hope”, united around people rather than around data or molecules.
Beyond just the patient groups, the collaboration among scientists, clinicians, companies, and patient advocates was credited for accelerating progress from molecular insights to therapies for CDD. As a reflection to this collaboration, there were CDKL5 Forum Awards of Excellence granted to UCB Pharma as the company making a difference, Dr Kyle Fink from UC Davis for Lab of the Year, and Dr Massimiliano Bianchi for Champion of Progress, as well as several Junior Fellowship Awards.
The Forum opened with the voice of the patient, with Lili Hass, mom of Margot and co-Founder of CURE5 sharing her family story and “seizures are just the side dish; developmental challenges are the main course”. She asks scientists to look for more therapeutic benefits and to also look beyond drugs and into tools that can make life easier for families like hers, such as equipment, feeding innovations, and better ways to track data from home.
Dr Maria Luisa Tutino, in her dual role as mum of Elettra and protein scientist, gave a talk during the gala dinner and explained that science is not just experiments, it is people, connections and persistence. Allyson Berent from the FAST Angelman Foundation echoed these words for urgency and persistence, as another rare disease mum and scientist.
And Dr Katheryn Frame, mum of Kiera, gave the last presentation, as the Chair of the CDKL5 Alliance, talking about “a world of united hope” and highlighting the work being done by so many CDD patient organizations across the globe. Katheryn explained that to her, “united in hope means strength, determination, resilience, compassion and connection”.
Majid Jafar, dad of Alia and co-Founder of the Loulou Foundation, closed the meeting reminding us that the growth of the CDKL5 Alliance is a huge part to the strength of this collaboration. As in other editions of the Forum, he reflected on how we have progressed farther and faster than we ever thought, but never as far or as fast as families would want to. Majid asked the attendees to renew our dedication to developing cures for CDD, and invited us to meet in one year for the 2026 CDKL5 Forum in London, UK.
And that’s it for the 2025 CDKL5 Forum! where we learnt about unexpected CDKL5 biology that opens new doors for treatment, saw more families eligible for clinical trials than ever before, and where the progress in gene therapies for CDD felt more tangible and real than it has ever been. This was a great Forum.
I hope you enjoyed this summary, and I’ll see you in London.
Ana Mingorance, PhD
Disclaimer: This is my own summary and key learnings, and not an official text about the Forum by the Loulou Foundation. I write these texts with the parents of people with CDD in mind, so excuse also my lack of technical accuracy in parts.
REPASO DEL FORO CDKL5 2025
La decimoprimera edición del Foro CDKL5 tuvo lugar en Boston, los días 27 y 28 de octubre de 2025. El Foro es una reunión anual que organiza la Fundación Loulou y en la que científicos y miembros de la industria farmacéutica se reúnen con representantes de la comunidad de pacientes para repasar los últimos avances en el campo.
Este es un repaso para los grupos de pacientes de las principales novedades del Foro CDKL5 2025.
Hace ya diez años que la Fundación Loulou organiza una reunión anual, el Foro CDKL5, donde los científicos de academia y de industria trabajando en el síndrome de deficiencia en CDKL5 (CDD), junto con representantes de los grupos de pacientes, se reúnen para compartir las últimas novedades y avanzar hacia tratamientos y una cura. Tenéis el resumen de los años pasados aquí: 2018, 2019, 2020, 2021, 2022, 2023 y 2024.
La edición de 2025 tuvo lugar en Boston los días 27 y 28 de octubre, y volverá a Londres para su edición de 2026. Este ha sido de nuevo el Foro más numeroso, con más de 200 participantes incluyendo representantes de 27 empresas y 22 grupos de pacientes.
Intentaré resumir las conclusiones principales de la edición de este año, no incluyendo todas las presentaciones sino centrándome en los temas principales y los progresos que vimos, que son muchos. Así que disculpas por adelantado a los científicos y médicos cuyas presentaciones no incluyo.
1. Ampliando aún más nuestro conocimiento sobre CDKL5
Fotos: Dr Dan Lavery (Fundación Loulou); Dra Enya Paschen (Ulysses Neuroscience); Prof Peter Kind (Universidad de Edimburgo); Imagen de algunas de las funciones clave de CDKL5; Resumen de pruebas de epilepsia en ratones con CDD en Ulysses Neuroscience.
Antes de este Foro 2025 yo pensaba que quizás habíamos averiguado ya todo lo principal sobre cómo funciona la quinasa CDKL5, y que por tanto los descubrimientos que faltan serían pocos y sin gran importancia. ¡Estaba equivocadísima! en este Foro oímos de varios descubrimientos que muestran que CDKL5 hace muchas más funciones que las que conocíamos, y muchas de esas son muy útiles a la hora de pensar en tratamientos.
Por ejemplo, científicos de dos países han descubierto a la vez que CDKL5 regula a una proteína que se une al RNA, lo que quiere decir que CDKL5 no solo enciende y apaga la actividad de otras proteínas (que es lo que hacen normalmente las quinasas) sino que también regula los niveles de otras proteínas. CDKL5 también estaría involucrada en autofagia, que es cómo la célula degrada agregados de proteínas. Así que de nueva esta es otra forma en la que CDKL5 estaría decidiendo lo mucho o poco que haya de otras proteínas en la célula. Y otro laboratorio ha descubierto que CDKL5 interacciona con varias proteínas sinápticas porque contiene un dominio tipo velcro que le permite pegarse a esas proteínas sinápticas (un dominio PDZ). Y esta información nueva hay que añadirla a las funciones importantes que ya sabíamos como el control de actividad del canal Cav2.3 o la modulación de los microtúbulos y la plasticidad neuronal.
Lo que esto significa, para científicos como yo que trabajamos en desarrollo de fármacos, es un mapa cada vez más amplio de posibles dianas terapéuticas. Así que aunque parezca que más complejidad es mala, en realidad es buenas noticias. También tuvimos una presentación de Ulysses Neurosciences que nos mostró su plataforma preclínica de evaluación de fármacos en ratones con CDD, en este caso tanto con ratones macho como hembras e incluyendo epilepsia y EEG. Es la plataforma más completa que existe.
En resumen: los científicos siguen descubriendo lo que se esconde en la biología de CDKL5, y cada uno de esos descubrimientos es una posible idea de cómo desarrollar tratamientos. Y el Foro 2025 estuvo particularmente lleno de nuevos descubrimientos de la biología de CDKL5.
2. Más opciones de entrar en ensayos que nunca hasta ahora
Fotos: lista de programas terapéuticos en desarrollo para CDD que presentó Dan Lavery; Prof Orrin Devinsky (Universidad de Nueva York); cómo funciona el fármaco de Angel Neurotherapeutics sobre el citoesqueleto; cómo funciona fenfluramina; lista de hospitales que tienen el ensayo clínico de bexicaserina (Lundbeck); ponente de Praxis el Dr Steve Petrou.
En su introducción, EL Dr Dan Lavery de la Fundación Loulou mostró una diapositiva de la lista de programas en desarrollo para tratar CDD, incluyendo varias generaciones de fármacos seguidos por varias generaciones de terapias génicas, todos avanzando hacia ensayos.
Y lo más importante es que en 2025 tenemos un fármaco aprobado, uno que ha completado con éxito el ensayo global de fase 3, y dos más que están reclutando ahora mismo en otras dos fases 3 globales. Jamás hubo un año con tantas opciones como 2025.
Este año por primera vez tuvimos a la empresa Immedica en el Foro, tras haber comprado a Marinus (que desarrolló la ganaxolona). El equipo de Immedica nos presentó cómo han aprendido que es mejor hacer la subida de dosis de ganaxolona más despacio que lo que pone la ficha oficial, para minimizar así los efectos secundarios. Ganaxolona ya está comercializada en EEUU y la empresa está trabajando para hacerla llegar al mercado en más países.
Este verano la empresa UCB Pharma anunciaba resultados positivos en su fase 3 de fenfluramina en CDD. En el Foro, nos explicaron en más detalle el complejo mecanismo de acción de fenfluramina, que va más allá que solo unirse al receptor de serotonina 5HT2C. Los resultados exactos de esa fase 3 nos los contarán en diciembre, en el congreso de la Sociedad Americana de Epilepsia, con lo que estaremos pendientes. Y UCB va a pedir la ampliación de la indicación terapéutica de fenfluramina para incluir también el tratamiento de las crisis en CDD.
El año pasado, las agencias regulatorias permitieron por primera vez que las empresas hicieran ensayos clínicos juntando los diferentes síndromes con epilepsia. Hasta entonces había que hacer los ensayos en cada síndrome por separado. Y esto nos ha llevado a tener más opciones de ensayos clínicos para CDD. Además, como estos ensayos incluyen niños y adultos y solo piden 4 crisis contables al mes, hay más personas con CDD que nunca antes que cualifican para estos ensayos y que pueden acceder a estos fármacos experimentales para la epilepsia. En el Foro vimos la presentación de dos ensayos globales de fase 3 que están ahora mismo reclutando en muchos países:
Lundbeck está reclutando para su ensayo de fase 3 DEEP OCEAN con bexicaserina. Bexicaserina usa uno de los receptores de fenfluramine, el 5HT2C, y tuvo resultados muy positivos en la fase 2 que también era de síndromes mezclados. Tienen unos 100 hospitales de EEUU, Australia, Asia y Europa (incluida España) en el ensayo, y el enlace de su ensayo es este: https://deepdeestudy.com/
Praxis está reclutando para su ensayo de fase 3 EMERALD con relutrigina. La relutrigina es un bloqueante de canales de sodio de última generación con muchas propiedades interesantes: no requiere ajuste de dosis (se empieza en la dosis buena), es solo una toma al día, tiene formulación líquida y para el ensayo no hace falta ir al hospital porque ellos te mandan un médico y una enfermera a casa en cada visita. El ensayo también está en muchos países (incluida España) y su web en 12 idiomas. El enlace de su ensayo es este: https://www.resiliencestudies.com/emerald
Además de esta primera generación de fármacos, que van todos dirigidos a reducir las crisis, pudimos ver un anticipo de lo que será la segura generación de fármacos: las medicinas de precisión. Las medicinas de precisión son aquellas dirigidas a aspectos clave de lo que está mal en neuronas con CDD. Las medicinas de precisión serían como un antibiótico para tratar una neumonía. Sigue siendo un fármaco al igual que el ibuprofeno, pero mientras que el ibuprofeno trata un síntoma (en este caso la fiebre), el antibiótico trata el problema de base. En el Foro vimos una presentación de Angel Neurotherapeutics, y su trabajo en ratones con CDD para desarrollar un fármaco llamado PME que corregiría el problema de microtúbulos que ocurre en ausencia de CDKL5. Y nos mostraban mejoras en los problemas de neurodesarrollo de los ratones con CDD, como no saber hacer un nido para dormir o tener problemas motores. Anticipan poder estar en ensayos en 2027.
En resumen: muchas más familias con CDD tienen opciones de entrar en ensayos que nunca hasta ahora. Y esto incluye a los pacientes adultos, que hasta ahora no entraban en casi ningún ensayo. En un par de años, esperamos empezar a tener ensayos clínicos con las medicinas de precisión.
3. Progreso en las terapias génicas, y una lección en toxicidad
Fotos: Prof Jim Wilson en su ponencia principal; diapositiva y ponente de la Fundación Loulou el Dr Russ Addis (terapia génica); ponente y diapositiva de la Universidad de California Davis el Dr Kyle Fink (terapia génica de reactivación del cromosoma X); ponente Andrew Steinsapir (Apertura Gene Therapy)
El tema de las terapias génicas sigue siendo el principal del Foro CDKL5, y este año recibimos tanto mensajes optimistas como mensajes de tener cuidado. Algunos miembros de la Alianza CDKL5 con los que hablé lo definían como que “por fin parece que vamos en serio”, y cómo este año el progreso que ha habido en el desarrollo de terapias génicas para CDD les ha hecho verlo como una realidad más tangible que nunca.
Tuvimos presentaciones de no una sino TRES de las diferentes terapias génicas que hay en desarrollo para CDD:
1. La Dra Sharyl Fyffe-Maricich de Ultragenyx habló de su terapia génica empezando por responder la gran pregunta, que es que aún no pueden decirnos cuando llegarían a ensayos clínicos. Pero nos dio un gran regalo científico al presentar su investigación en monos que muestra que las neuronas que ya tienen CDKL5 aún tienen la capacidad de producir más si se les da una terapia génica. Esto es importante para las muchas personas con CDD que tienen mutaciones missense, que son las que cambian un aminoácido de la proteína por otra, con lo que tienen CDKL5 que no funciona pero que está ahí. La investigación de Sharyl apunta a que al final se produciría una mezcla de proteínas CDKL5 en la neurona, algunas viviendo del gen que está mutado y algunas de la terapia génica, lo cual es muy buenas noticias. Así que me llevé ese alivio y gratitud a Ultragenyx por contarnos ese avance de conocimiento.
2. El Dr Russ Addis de la Fundación Loulou presentó por fin una actualización de cómo va la terapia génica para CDD que está desarrollando la Fundación. Esta terapia génica la están (estamos) haciendo en colaboración con el Profesor Jim Wilson, usando un virus que es muy parecido al AAV9 y que ya está en otros 4 ensayos clínicos ahora mismo. Y lo que lleva ese virus dentro es una copia del gen CDKL5, que se administra directo al líquido cefalorraquídeo (por la nuca). Russ nos enseñó los experimentos en ratones para dar con la dosis correcta y nos dijo que ya han terminado todos los experimentos de toxicología en primates, con lo que han comenzado la producción del virus para avanzar a ensayos. Esta parte tarda, nos dijo, “más o menos un año”, con lo que espera pedir la aprobación de ensayos a final de 2026 para empezar los tratamientos en 2027. La Fundación Loulou anunció una colaboración con la empresa Gemma Bio de Jim Wilson para compartir los datos de esos otros ensayos clínicos de terapia génica que usan un diseño muy igual a esta. Y para avanzar este y otros proyectos, la Fundación Loulou ha creado una biotecnológica llamada Elaaj Bio, propiedad única de la Fundación, con la visión de “que las familias y lo afectados tengan más de una opción terapéutica, y más opciones de acceder a todas esas terapias”.
3. El Dr Kyle Fink, de la Universidad de California Davis, está desarrollando la famosa terapia génica de reactivación del segundo cromosoma X. Esta terapia funciona también usando un virus, pero en vez de llevar el gen CDKL5 dentro lo que lleva son las instrucciones para leer la segunda copia del gen CDKL5 que todas las niñas y mujeres tenemos en nuestro segundo cromosoma X. Esto lo consigue usando una variante de CRISPR. Ya en otros años nos había mostrado lo bien que funciona esta terapia en los ratones con CDD, y este año nos vino a contar su “progreso convirtiendo la ciencia en medicina”. El nuevo progreso incluye también identificar la mejor dosis para usar, y comprobando que funciona si la neurona es humana y no de ratón, usando organoides a partir de células pluripotentes de pacientes. En este caso están comenzando los estudios de toxicidad en monos y la producción del virus para ensayos todo en paralelo, porque ya la terapia génica está optimizada tal cual está. No dio fechas, pero si que nos explicó que su colega el Dr Julian Halmai está trabajando en una variante de CRISPR en miniatura que les permita meter toda esta terapia en un virus solo, ya que actualmente utilizaría dos tipos de virus (uno con la primera mitad del CRISPR y otro con la segunda, que luego se encuentran en cada neurona).
También pudimos oír de una terapia génica que ha sido parada:
Esta fue una sorpresa interesante. Ashron Brennecke de Biogen, subió al pódium para explicarnos que Biogen había estado trabajando también en una terapia génica para CDD en secreto, pero que habían decidido eliminar el programa. Ahora que ya no están trabajando en CDD, querían compartir con nosotros el método para medir eficacia de la terapia en ratones usando EEG y que creen que se podría usar también en ensayos clínicos. Estuvo interesante oír ese trabajo, pero a la vez es bastante triste saber que ya van tres, y que Biogen se une a Amicus y a PTC Therapeutics como empresas que empezaron pero luego abandonaron el desarrollo de terapias génicas para CDD. Pero al fin y al cabo esto es ciencia y esto son empresas, con lo que abandonar proyectos por el camino está a la orden del día y es justamente la razón por la que debemos tener varias opciones en desarrollo en todo momento.
Y un tema muy importante, pudimos aprender de las lecciones que nos ha dejado el campo de la terapia génica desde que la primera persona recibiera la primera terapia en los años 90:
El Profesor Jim Wilson ha sido y es uno de los pioneros del desarrollo de terapias génicas, y nos dio una ponencia espectacular sobre lo que han sido estos 30 años y lo que han aprendido. Jim es el antiguo Director del Orphan Disease Center de la Universidad de Pensilvania, y el actual CEO de Gemma Bio – la empresa de terapias génicas que está trabajando con la Fundación Loulou en la terapia para CDD. Jim nos explicó cómo el primer problema que afrontaron fue que para conseguir terapias génicas tendrían que pelearse con el sistema inmune, y que les llevó 17 años (que se dice pronto) entender bien qué había pasado en los primeros casos de fallecimientos por terapias génicas. Jim es el científico que descubrió AAV9, el virus más famoso, y ya ha llevado a ensayos muchas terapias génicas usando el AAV9 o su nueva generación de virus. Jim dedicó la última parte de su ponencia a un tema del que se habla mucho ahora en enfermedades raras: los recientes casos de fallecimientos como consecuencia de la administración intravenosa de AAV en dosis altas. Su laboratorio lleva estudiando la toxicidad de las terapias génicas en caso 500 monos, y lo que Jim pide es transparencia a todas las empresas trabajando en terapias génicas para que cuando ocurra un caso de fallecimiento en un ensayo compartan con la comunidad científica exactamente qué pasó. Porque solo así podrán Jim y otros científicos desarrollar medidas para ver esa toxicidad en los estudios en monos previos a ensayos, y evitar así más fallecimientos.
Andrew Steinsapir, de la empresa Apertura, nos habló de su nuevo virus diseñado para ser administrado por vía intravenosa y que cruce la barrera hematoencefálica aprovechando unos receptores de la superficie vascular cerebral. Ya existen otras terapias que usan macromoléculas (aunque no virus) que pasan de sangre a cerebro usando esos mismos receptores, con lo que el equipo de Apertura considera que su virus no tendrá problemas de seguridad cuando llegue a ensayos. Y la verdad es que si este tipo de virus resulta ser seguro en ensayos clínicos nos permitiría tener una segunda generación de terapias génicas para CDD que en vez de requerir a un neurocirujano para inyectarla en el líquido cefalorraquídeo a través de la nuca solo requiera una infusión intravenosa.
Y por último el Dr Basel Assaf, un toxicólogo brillante que trabaja para la empresa Attentive Science, se hacía eco de las palabras de Jim diciendo que hasta ahora hemos podido anticipar en monos cualquier problema que luego apareció en la clínica. Sin embargo esta predicción no ha sido perfecta, porque recientemente nos hemos llevado una muy mala sorpresa con una terapia génica para otros síndrome de desarrollo en la que tuvieron una muerte en el ensayo mientras que los monos no habían dado ninguna señal. Era de nuevo un caso de dosis alta intravenosa, y Basel nos ayudó a hacernos una imagen mental de qué queremos decir con dosis alta de virus: una infusión intravenosa de terapia génica a dosis altas usa siete cuatrillones de virus, que son más que todas las células de nuestro cuerpo.
Por cierto que os recuerdo que las terapias génicas para CDD de Ultragenyx, la Fundación Loulou y la Universidad de California Davis, no usan ni dosis altas ni vía intravenosa. Usan todas dosis más bajas y van directas al líquido cefalorraquídeo, no por via venosa.
En resumen: la medicina está aprendiendo cómo funcionan los riesgos de las terapias génicas, lo cual requiere transparencia y colaboración. Tenemos varias terapias génicas en desarrollo para CDD con la posibilidad de ensayos clínicos en 2027. El mensaje general de la sesión del Foro sobre terapias génicas fue bastante positivo. Como un papá de CDKL5 puso en redes sociales, “se viene un cambio importante".
4. Posiblemente ya estemos listos para hacer ensayos clínicos más complejos
Foto: el panel de la sesión de escalas clínicas, con el Dr Xavier Ligioer (Fundación Loulou), el Dr Barry Ticho (Stoke Therapeutics), la Dra Heather Olson (Hospital Infantil de Boston), la Dra Jenny Downs (Kids Research Institute en Australia), falta en la imagen el Prof Tim Benke (Universidad de Colorado) y por último el ex-Director de neurología de la FDA actualmente con la Fundación Loulou el Dr Billy Dunn.
Durante los últimos 5 años o así hemos estado hablando de la necesidad de estar preparados para ensayos clínicos en CDD que miren más que las crisis epilépticas. Y cómo para eso hay que tener escalas clínicas aprobadas por reguladores y validadas para medir los otros aspectos principales de la enfermedad. Tras ver las presentaciones del Foro de este año yo creo que ya estamos listos, y que si tuviéramos que empezar mañana un ensayo clínico con una terapia génica sabríamos qué medidas usar.
El Dr Xavier Liogier de la Fundación Loulou presentó las últimas noticias del estudio observacional CANDID, en el cual en España tenemos muchas familias participando. CANDID usa escalas clínicas que ya se han usado para aprobaciones de tratamientos en otras enfermedades y que cubren los aspectos principales de CDD también. Se espera que en diciembre de este año lleguen 100 pacientes a la visita d ellos 2 años del estudio, y ya sabemos cuáles de las escalas funcionarían en ensayos y hay un manuscrito científico enviado a publicación. Una de las lecciones de CANDID, con implicación para ensayos clínicos, es que de promedio las personas con CDD tienen entre 1 y 5 crisis epilépticas contables al día. ¡Al día! con lo que la inmensa mayoría podrían participar en los ensayos clínicos que hay abiertos en este momento, porque es mucho más que en otros síndromes con epilepsia.
El Profesor Tim Benke, de la Universidad de Colorado, y la Dra Jenny Downs del Kids Research Institute en Australia, presentaron su trabajo para desarrollar nuevas escalas clínicas, específicamente diseñadas para CDD. El estudio observacional que lidera Tim también está a punto de llegar a 100 participantes con seguimiento de 2 años, y este estudio además incluye medidas de EEG con lo que podría identificar una medida electrofisiológica para usar en ensayos clínicos y ver qué está cambiando en el cerebro más directamente. Jenny está desarrollando una escala específicamente para medir la comunicación en CDD, que es muy muy importante para las familias, y que recibió los elogios de un antiguo director de la FDA por el trabajo tan detallista y diligente que están haciendo con esta escala.
En un panel de debate que cerró esta sesión, los panelistas, incluyendo ese antiguo director de la FDA el Dr Billy Dunn y el Director Médico de la empresa Stoke Therapeutics Dr Barry Ticho, señalaron que los ensayos clínicos y el cuidado médico de los pacientes tienen diferentes objetivos. Si bien durante el cuidado médico de un paciente hay que mirar a todos los síntomas y a los que más impactan a ese paciente en concreto, “no hay que mostrar todo lo habido y por haber en un ensayo para obtener una aprobación”. Billy advertía de evitar esto para “no crear obstáculos sin querer” que pudieran complicar los ensayos clínicos. En vez de sobrecargar los ensayos, recomendaba que una vez esté el fármaco aprobado ya los médicos miren todos los escenarios en los que podría funcionar ese fármaco, además del beneficio para cada paciente de forma individual. Pero todo eso una vez están ya aprobados.
En resumen: Hubo mucho apoyo desde la industria y profesionales reguladores al trabajo que llevan haciendo estos equipos para desarrollar y validar escalas clínicas para CDD que midan más allá de las crisis. Y estos estudios son solamente posibles gracias a la generosidad de cientos de familias que llevan asistiendo dos años (o algunas más) a las visitas de estos estudios, donde no reciben ningún tipo de fármaco. ¡Muchísimas gracias a todos los que hacéis esto posible!
5. Una comunidad global, y una alianza de esperanza
Fotos: Mensaje y ponencia de Lili Hass (CURE5); mensaje de la Dra Maria Luisa Tutino; foto de Maria Luisa con Lynn y Majid Jafar; mensaje y foto de la Dra Katheryn Frame (Alianza CDKL5).
Durante el Foro, varias veces se refirieron a la comunidad de pacientes internacional, y sobre todo a los países que son miembros de la Alianza, como “una alianza global de esperanza”, unida en torno a las personas más que en torno a proyectos o moléculas.
Más allá de los grupos de pacientes, la colaboración entre científicos, médicos, empresas y representantes de familias es la que ha conseguido acelerar el progreso de los descubrimientos científicos a tratamientos para CDD. Reflejando esta colaboración, en el Foro se dieron premios a la empresa UCB Pharma como empresa que ha marcado una diferencia en CDD, al Dr Kyle Fink como laboratorio del año, y al Dr Massimiliano Bianchi como promotor de progreso, todos por sus contribuciones recientes a la enfermedad. También se dieron premios a jóvenes científicos que están realizando sus proyectos en CDD.
EL Foro siempre abre con la voz del paciente, en este caso con Lili Hass, la mamá de Margot y cofundadora de CURE5. Lili explicó a la sala que “las crisis son solo el aperitivo en CDD, el plato fuerte son los problemas de neurodesarrollo”, y pidió a los científicos que busquen soluciones más allá que con fármacos, por ejemplo con equipamiento, facilitar la alimentación o facilitar la captura de datos desde casa.
La Dra Maria Luisa Tutino, mamá de Elettra y científica, nos dio una presentación durante la cena de gala explicando que la ciencia no son experimentos: es gente, es conexiones, y es perseverancia. Recibimos un mensaje similar de Allyson Berent de la fundación de síndrome de Angelman FAST, que es también madre y científico.
La Dra Katheryn Frame, mdare de Kiera, dio la última ponencia, en capacidad de presidenta de la Alianza Internacional CDKL5. Su mensaje fue de “un planeta unido en la esperanza” y destacó la labor de muchos de los grupos que son miembros de la alianza. Katheryn explicó que para ella “unidos en la esperanza significa fuerza, determinación, resiliencia, compasión y conexión”.
Majid Jafar, el padre de Alia y co-Fundador de la Fundación Loulou, cerraba el congreso recordándonos que el crecimiento de la Alianza CDKL5 es el elemento central que da fuerza a esta colaboración entre científicos, médicos y familias. Como en ediciones anteriores del Foro, hacía la reflexión de que hemos llegado más lejos y más rápido de lo que pensábamos, pero nunca es suficientemente lejos ni suficientemente rápido para lo que querrían las familias. Majid pidió a los asistentes renovar su compromiso para desarrollar curas para CDD, y nos invitó a reunirnos de nuevo en un año para el Foro 2026, esta vez en Londres.
¡Y hasta aquí llegó este resumen del Foro CDKL5 de 2025! donde aprendimos de biología que no podíamos imaginar y que abre puertas a nuevos tratamientos, supimos que había más familias con posibilidades de entrar en ensayos que nunca antes, y donde el progreso en las terapias génicas para CDD se volvió más tangible e inmediato que nunca. El de 2025 ha sido muy buen Foro
Espero que os haya gustado este resumen, y nos vemos en Londres.
Ana Mingorance, PhD
Nota: este texto captura mis impresiones de las presentaciones del Foro que más me interesaron, no es un texto oficial del congreso emitido por la Fundación Loulou. Escribo estos resúmenes para los padres de personas con CDD, así que a veces me tomo ciertas licencias a la hora de explicar las partes mas técnicas.
CDKL5 ALL IN(VOLVED) 2025
This is a scientific summary of the 2025 CDKL5 Alliance patient conference celebrated in Rome. The CDKL5 Alliance is the umbrella organization bringing together national CDKL5 deficiency patient associations as well as affiliated organizations like the Loulou Foundation. The summary covers the scientific updates presented during the conference in June 2025.
1. Intro: Back in Rome
The first CDKL5 conference that I attended happened to be the patient meeting in Rome in 2017, where the CDKL5 Alliance was started. I am a neuroscientist and I was already working with patient foundations for related disorders, so I wanted to learn about CDD. I remember meeting some Spanish families (my country) who quickly explained me the disease. Also seeing Marinus on stage talking about a very small trial that they were running in CDD with their experimental drug ganaxolone. And I remember Antonino Caridi, a CDD grandpa, who took the initial leadership of the patient Alliance and welcomed us from stage.
I ended up joining the Loulou Foundation in 2018, so having the CDKL5 Alliance meeting return to Rome in 2025 felt very special. We were back in the same place, but everything about CDD is now immensely larger: the size of the community, the treatment pipeline, and the number of people working on it. And Antonino still looks the same.
The ALL IN(VOLVED) conference started Friday June 27th, with families catching up with each other, and meetings of the national patient group leaders and also with the pharma representatives. To give you some numbers, we had over 300 participants, with 41 people with CDD and many siblings who made the conference very enjoyable. In total we had participants from 24 countries or territories! in alphabetic order: Albania, Australia, Austria, Belgium, Brazil, Bulgaria, France, Germany, India, Ireland, Italy, Japan, MENA, Netherlands, Philippines, Poland, Romania, Slovakia, Spain, Sweden, Switzerland, the UK, Ukraine and the USA. That’s why we call it the Global Alliance. And we had 9 pharmaceutical and biotech companies supporting the meeting. Just hearing these numbers would have blown the minds of the 2017 participants.
Then on Saturday 28th we started with a series of science talks for all attendees, where we reviewed the disease, the natural history studies, the pipeline, and learnt about different therapeutic modalities and where they are. We called these the ALL IN(FORMED) sessions.
We also had a presentation from the team at the Pediatric Therapy Center High Hopes, in Dubai, because the non-pharmacological therapies are so far the best therapies that we have for kids and adults with CDD. They showed us best practices to improve strength and mobility and promote neuroplasticity in these kids, and talked about a “multidisciplinary team with a family-centric approach” with parents as co-therapists.
For the afternoon we split into two tracks. I stayed with the scientists and the pharma companies in a partnering session where we learnt about specific programs on new therapies and model systems for the disease. I will mention these in my summaries below but without much detail, given that this track was designed to be only for scientists and not open to families. The parallel track for families included biomarker sampling and pet therapy with a dozen of beautiful dogs, which made us, scientists, very jealous.
And we closed the conference on Sunday June 29th with the ALL IN 1 COMMUNITY session where the families took the stage and we all listened.
I will summarize below the main news from the scientific sessions, and some of the main messages from the families that I found particularly impactful. Please keep in mind that I am looking at the therapeutic progress as a scientist, not as a parent. And this is not the entire summary of the ALL IN(VOLVED) CDKL5 Alliance conference, so apologies in advance for the parts that I might be missing.
2. Science: small molecule drugs
The first generation of treatments developed for CDD are anti-seizure drugs. That’s because CDD is one of the genetic syndromes that comes with the highest seizure frequency, and there is no good drug to control those seizures, so we really need something better.
In 2017 in Rome, Marinus presented data on 4 patients with CDD taking ganaxolone, FOUR. Three out of four had improved, the fourth one didn’t, but it looked promising. Their plan was to recruit more patients.
By the time we met back in Rome for the ALL IN(VOLVED) 2025 conference, we know that the Phase 2 trial included 7 patients and was successful, that it would be followed by a global Phase 3 trial with more than 100 patients also successful, that then Marinus succeeded at getting ganaxolone approved for treating seizures in CDD in the US and Europe, and that Marinus was later acquired by a larger company called Immedica Pharma who was now with us in Rome at the conference. Change and progress can seem slow looking forward, but quite fast when you look backwards.
Carol-Anne Partridge is a CDD mum (and leader of the UK patient group) and she presented a study designed to convey better to governments and payers the severity of CDD. This is very important because ganaxolone is the first drug approved for CDD, so when authorities evaluate their reimbursement decision on ganaxolone they are learning about the drug AND the disease at the same time. And because of this learning curve, ganaxolone is still only commercially available in the US. The study surveyed 132 families, and showed that 96.7% of patients are taking anti-seizure drugs, and that if you measure the health-related quality of life of the CDD patients as reported by their parents from 0 (a state comparable to being dead) to 1 (representing full health), the average is 0.18. The survey used the methodology that payers want to see, and was designed to “speak” to them. I think the message is loud and clear about the need for new medications.
The biggest news of this conference came from our second Phase 3 trial in CDD: UCB Pharma issued a press released the first day of the conference announcing that the Phase 3 trial with fenfluramine for treating seizures in CDD was successful, both in efficacy and safety, and that they will be moving forward to request an approval for CDD now (fenfluramine is already approved for two other syndromes). If everything goes well, this will become the second drug approved for CDD. And thank you UCB for the perfect timing with the announcement, we all got to celebrate together.
And what comes after two global Phase 3 trials? another global Phase 3 trial. The company Longboard (part of Lundbeck after an acquisition) is developing a second-generation fenfluramine, called bexicaserin, that is already in Phase 3 trials in the US and is adding many more countries world-wide progressively this year. Interestingly this drug is bringing together into the same trial all of the rare epilepsy syndromes, that are often called “DEEs”, and because people with CDD often have many seizures it means that many will qualify for this trial. It asks for a minimum of 4 countable seizures a month, and ages 2 to 65. In Rome, we had Dr Marina Trivisano present an update on this program and show us the map with all the countries in the trial. You can see more information online if you look for the “DEEp OCEAN” trial, and I encourage you to ask your neurologist about it if you are interested.
These first three large trials were for seizure medications, but I also explained in my presentation about the CDD pipeline that after these trials we are moving into therapies that treat the disease, not the symptom. And this includes not only the gene therapies (more about this in a minute) but also small molecule drugs that act on the most important targets of CDKL5. What we call “small molecule drugs” are the regular medicines that are taken orally like a pill or a syrup and that are made of a chemical compound – not something big like a virus or a protein. And because the work of the CDKL5 protein in the cell is to turn on or off other proteins, acting like a switch (that’s what kinases do), it is possible for scientists to design small molecule drugs that can bind to those proteins now, and turn them on or off as they needed.
In the morning session I introduced that concept, and I called it precision medicine because those drugs are designed to correct some of the key targets of CDKL5. And later in the afternoon during the partnering session we had a presentation from Prof. Massimiliano Bianchi who is developing one of those precision medicine approaches, with drugs that target the cytoskeleton (more of why that’s important for CDKL5 HERE in section 1).
3. Science: gene therapies
There are several gene therapies in development to treat CDD, and we hope that they will get to clinical trials in the near future. A gene therapy uses a virus to deliver a copy of the CDKL5 gene to the brain, so it is made of the outside of a virus (to help it get into brain cells) but inside it doesn’t have virus DNA, it has the CDKL5 gene.
Dr. Stuart Cobb is one of the inventors of a gene therapy for Rett syndrome (with the gene MECP2) and gave us a beautiful lecture on how gene therapies work. In his words: “it is an easy concept, but with complicated execution”. It essentially follows four steps: make the virus, cure a mouse, check for toxicity in monkeys, and then go to clinical trials. He explained how in Rett syndrome they are already in clinical trials, and they are seeing clinical improvements. And two days after his talk, the company developing his gene therapy announced that they are progressing to Phase 3 trials with their gene therapy in Rett syndrome.
Some years ago ,we thought that gene therapies for CDD would be in clinical trials by now. Ultragenyx had said at the 2022 CDKL5 Forum that they hoped to be in trials in the following year. But that hasn’t happened yet, two and a half years later, so I know that has disappointed many families who have been counting how old their kids will be by the time the gene therapies arrive to trials. And that premature announcement has probably also made the other gene therapy developers refrain from speaking too soon about their timelines, to not risk disappointing the community once again. As a result, we are still a bit in the dark about how many gene therapies are being developed for CDD and how far along they are.
Majid Jafar and I (both from the Loulou Foundation) mentioned in our presentations that there are multiple gene therapies in development for CDD, but it would have been much better to get some of the companies present an update. Even without stating timelines, seeing the progress that they are making would have been and influx of air for the 300+ family members in the room.
We did have two presentations dedicated to a special type of gene therapy for CDD: a gene therapy designed to open up the second CDKL5 gene copy. For background: because males only have one X chromosome, female cells only use one of our X chromosomes and turn the second one into a tiny little ball that is not read. That way, both males and females have one functional X chromosome. Which X chromosome gets inactivated is totally random and is different for each cell. What this gene therapy does is to carry inside of a virus the instructions to read the second copy of CDKL5, the one in the inactive X chromosome. And because girls with CDD have always one good CDKL5 gene copy (the other one is mutated) this gene therapy can help each of their cells read the two copies, one of which is good, and that’s all we always needed! Dr. Kyle Fink is leading this program and told us that they are now making “clinical grade gene therapy” to start the safety experiments in monkeys that come prior to trials.
4. Science: Enzyme Replacement Therapies
We also had a lecture on how enzyme replacement therapies work. Prof. Elisabetta Ciani explained very nicely how an enzyme replacement therapy consists of making “lab-made CDKL5 protein” to then give it to neurons. This is a good idea because CDKL5 is an enzyme, and other brain enzymes have been successfully adapted to enzyme replacement therapies. But as Stuart had said earlier that morning, this is also “an easy concept, but with complicated execution”. Elisabetta had succeeded at making a modified CDKL5 (so that it enters cells) in her lab and using it to treat CDD mice, but the same methods that worked for mice don’t work as a medicine for people, and here is where the challenge has been.
Prof Maria Luisa Tutino, who is both a CDD mum and a protein scientist, has been trying to make as many changes as needed to the CDKL5 protein to make it suitable as a lab-made protein therapy. The first-generation lab-made CDKL5 only goes to the “stomach” of neurons, so they eat it instead of using it, and she is currently working on a second-generation approach for which she has received a 1 million Euro grant. This one is clearly a very complicated therapeutic approach, but one worth fighting for.
5. Making it easier to develop treatments and to run trials
Before getting to trials, we need to believe that the treatment is going to work. How do we check that it could work? by testing it first in animals with the disease. And once we get to trials, we need to confirm that the treatment works, this time in patients. And how do we see if it worked in patients? by measuring clinical changes.
At the 2025 patient conference we got updates on animal models and on clinical change measures.
We reviewed the animal models in the partnering session, with Prof. Leonor Cancela showing us the zebrafish model of CDD (and small molecule screenings that she is running), María del Carmen Martín showing us the drosophila model of CDD (which has lots of seizures) and the Ulysses Neuroscience team showing us their CDD mouse model testing service. That means we have the entire toolbox to run preclinical trials in CDD animals.
And we got updates on the studies to measure clinical changes during the big morning session with all families in the audience, because all CDD families are critical players in these clinical programs.
Prof. Tim Benke gave us an update about the disease, and also updated us about the ICCRN natural history study in the US where they are developing a single scale to measure the global severity of CDD for each patient. And Dr. Xavier Liogier told us about the international CANDID study that is managed by the Loulou Foundation, where we have been able to see that there are many seizures at all ages, that most of the developmental improvements are seen in children under the age of 6, and that there are several scales to measure different disease domains (like cognition, communication, behavior or motor skills) that could be already used in trials. These two studies have enrolled more than 200 patients together, and participating in these studies means several hospitals visits over several years, so it is a big effort by the patients and their families and it would be impossible to design complex clinical trials (like for gene therapies) without all of you.
While the natural history studies help us know how to measure EXTERNALLY how a medicine might be helping, we use biomarkers to measure the INTERNAL changes. At the Rome conference we saw an update on the ELPIS global biomarker study by Prof. Massimiliano Bianchi, where they track changes in proteins in blood that tell us what is happening in the brain. And we also had a presentation by Vita Cardinale, who won the conference poster competition and was invited to give a talk on her project where they look at biomarkers in saliva from patients. These biomarker studies are made possible by more than 100 volunteer patients and their families who are helping these scientists identify the internal measures of change.
By the way you might wonder how blood or saliva can tell us what is happening in the brain. The answer is that neurons talk to each other by sending out little bits of neuron that contain proteins and RNAs almost like sending a letter by post to each other. The inside of those little bits tells the recipient how that original neuron was feeling. Sometimes the recipient is a neighbor neuron, but sometimes those letters travel so far that the recipient is a scientist that can pick it up in liquids like blood or saliva and read that letter. Science can be very cool.
6. Voice of the community
Patient conferences can be very emotional, with many highs and lows. In Rome we shared three days with families in very different situations, from those who had their little kids with CDD taking their first steps, to parents who have lost their children. So it was only fair that after the main conference day families took the stage.
During this final session we listed to #1minuteofhope videos where parents from all over the world told us what hope meant for them, and asked the audience “what does hope mean for you?”. We had videos from Italy, Peru, USA, Canada, Japan, Ukraine, MENA, and France.
Mais Kanan from CDKL5 MENA started the day, and Majid Jafar from the Loulou Foundation gave us a recap for how much progress has happened since his first daughter Alia got diagnosed with CDD in 2014. His was a message of hope, hard collective work that got us to where we are, and perseverance to get where we need to be.
We then got to hear from two siblings, starting with Iman Jafar who told us about how much she loves having Alia as her older sister, and how the first word that she ever spoke was “up” after hearing so often everyone tell “up!” to Alia to help her with her motor skills. And later we heard from Alessandro Caridi, who had a harder story as a sibling to a sister with CDD, but who also reminds us that the experience of every family and every person is unique, and to not underestimate the impact of this severe disorder on everyone around.
Dr Michela Fagiolini spoke about the possible future and dreams, from her experience working as a scientist in CDD and related diseases. She had some really good messages for families, like “drugs tailored for CDD and gene therapy are coming. These are not just abstract hopes, they are real and moving forward”. And how “it cannot be one person or one lab or one family, everyone is needed, and you are not alone”.
Then CDKL5 Spain shared two voices, one from a founder parent, and one from a more recently diagnosed family. Sandra Pérez spoke about being one of the “5 families and 5 stories” who created the Spanish association in 2014, and how one of the main missions of the group is and was to welcome new families, because they know how it feels to receive a diagnosis that changes everything, and how important to know that you are not alone. Then Manuel Vigara spoke about how thankful he was to those first 5 families who were “a light in the middle of dark, and whose steps opened our path”. He explained that the word “rare” often means no resources and no answers, and asked for the science community to keep dreaming and progressing, because “each hour in the lab is a thread of hope for millions of families” and “each small progress is a miracle in the making”.
We heard from the Fondazione Telethon in Italy about their support to rare genetic patient groups, and how “tenacity” is the identity of the Italian association and host of this meeting, CDKL5 Insieme Verso la Cura.
Last Barbara Verdirame, president of CDKL5 Insieme Verso la Cura, took the stage to thank everybody, from the families to the volunteers to the scientists and the doctors and the industry representatives in the room and everyone who supported the conference. Because in the end it is only through that massive collaborative effort that we get to advance medicine.
And I want to close this summary by capturing some words from Antonino Caridi, who opened the conference by reminding us of how far the International CDKL5 Alliance has come since it was founded in Rome in 2017, how “hope” is not a passive word, but one that gives us the courage to transform the injustice of CDD, and that “the best is yet to come”.
Ciao a tutti!
Ana Mingorance, PhD
Disclaimer: I write these texts with the parents of people with rare epilepsy syndromes in mind, so excuse also my lack of technical accuracy in parts. And credit for the beautiful pictures goes to Massimiliano Marcoccia and the great team from CDHL5 Insieme verso la cura.