Dravet syndrome pipeline review 2020 now available
Dravet syndrome pipeline and opportunities review 2020 is a market research publication that provides an overview of the global therapeutic landscape of Dravet syndrome, an orphan epilepsy disorder with multiple non-seizure comorbidities and high unmet medical need.
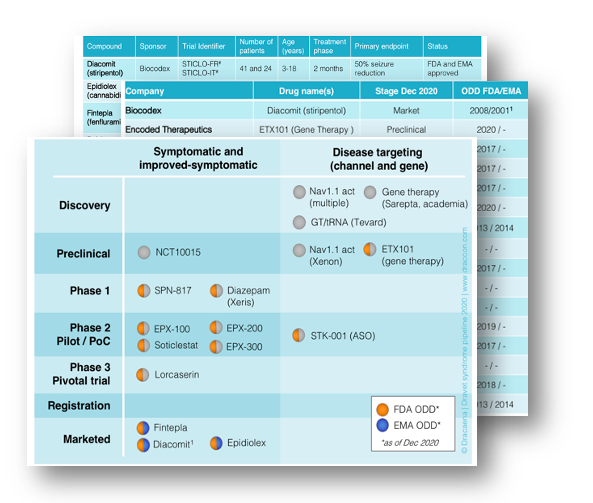
As of end 2020, the Dravet syndrome pipeline comprises 3 approved drugs, 11 drug candidates, and 12 different products have received orphan drug designations. Since the last report the pipeline has changed by, among others, the approval by FDA and EMA of Epidyolex (cannabidiol) and Fintepla (fenfluramine), the initiation of the first clinical trial with an antisense therapy, and progresses in different disease-targeting modalities including gene therapy approaches.
The 2020 pipeline report includes the most recent updates on programs from Biocodex (stiripentol), Encoded Therapeutics (ETX101), Eisai (lorcaserin), Epygenix Therapeutics (EPK-100, -200 and -300), GW Pharmaceuticals (Epidiolex / Epidyolex), INSYS Therapeutics, Lundbeck, NeuroCycle (NCT10015), OPKO Health (OPK88001, CUR-1915), Ovid Therapeutics (Soticlestat, OV935), PTC Therapeutics (ataluren), Sarepta, StrideBio, Stoke Therapeutics (STK-001), Supernus Pharmaceuticals (SPN-817, Huperzine), Takeda Pharmaceutical (Soticlestat, TAK-935), Tevard Biosciences, Xeris Pharmaceuticals (XP-0863), Zogenix (Fintepla, ZX008) and Zynerba Pharmaceuticals.
The Dravet syndrome pipeline and opportunities review 2020 report also includes an analysis of the competitive landscape, unmet needs, and evaluates current and future opportunities of the Dravet syndrome market.
You can find the 2020 pipeline review, as well as the 2019, 2018 and 2017 editions here: