Epilepsy Insights
TOP 5 INSIGHTS FROM THE AMERICAN EPILEPSY SOCIETY MEETING 2023
The American Epilepsy Society (AES) meeting is the largest epilepsy meeting of the year, and because it takes place every month of December it also serves as an annual review on the understanding and treatment of epilepsies. These are my top 5 insights from the American Epilepsy Society 2023 meeting.
I often write a summary of the main lessons from the American Epilepsy Society meeting, but for a second year there was so much about rare epilepsy syndromes or DEEs that I was not able to follow the main conference. I only went to one lecture session, and focused instead on the posters, scientific exhibits and meetings, which are all the opportunities to directly talk to people and by far the main value of this congress.
This is my summary of the trends that I am seeing in rare epilepsy syndromes after attending AES 2023. It is not a summary of the conference itself, which has really become too large to cover. In particular, I wanted to see if 2023 delivered on the promise set last year, which was Escape velocity for genetic epilepsy syndromes.
1 – EPILEPSY AS A COLLECTION OF RARE DISEASES
The larger pharma space has experienced a clear shift towards more personalized medicine and more orphan drugs. And epilepsy is not an exception. Since around 2014 with the clinical trials of cannabidiol, we have seen an explosion of programs directed to epilepsy syndromes, both with symptomatic and with gene-targeted approaches.
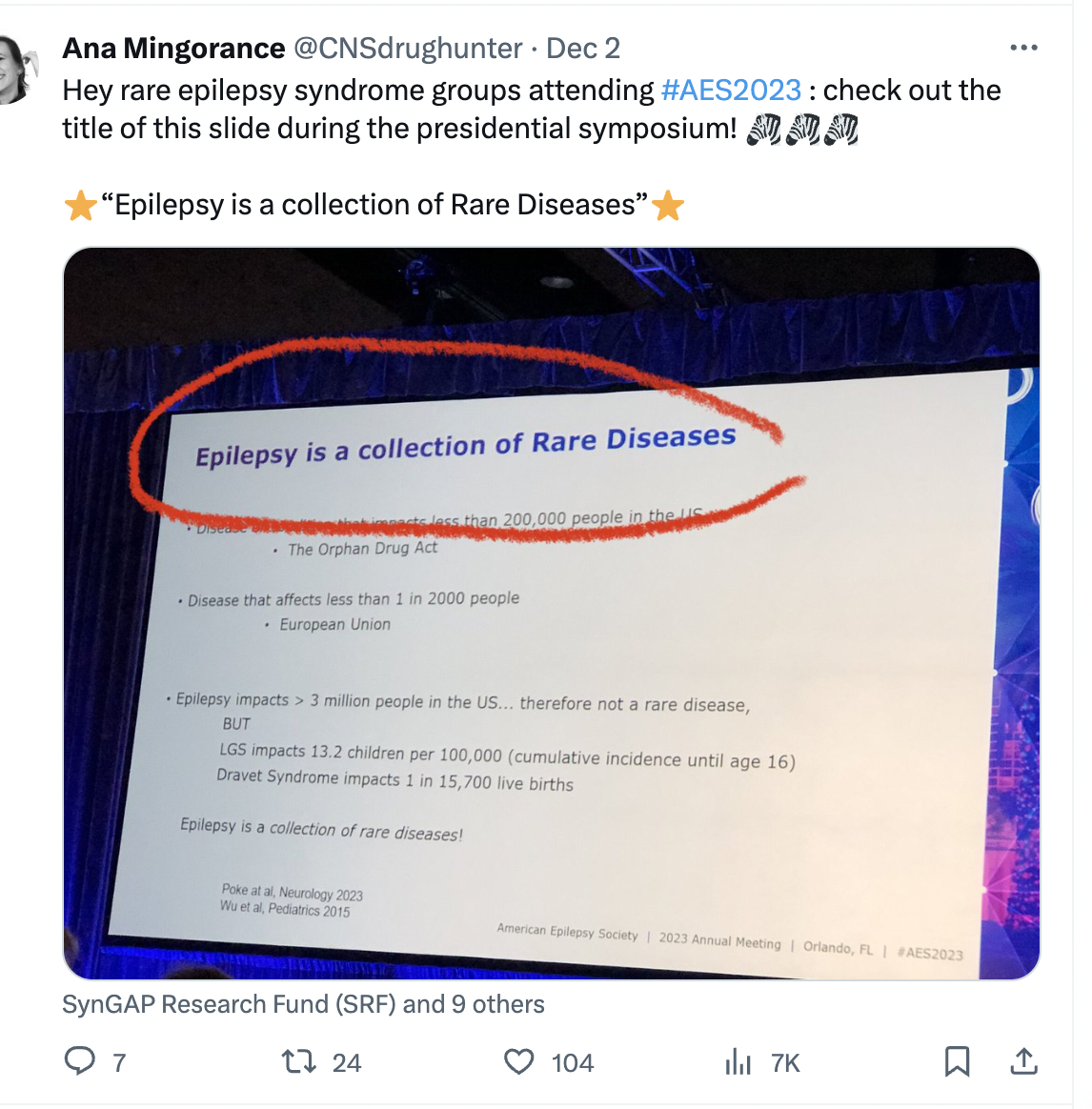
At AES 2023 I attended the plenary session where Dr Kelly Knupp from Children’s Hospital Colorado showed one slide with a key title, which ended up getting very popular on social media. It read: “Epilepsy is a collection of Rare Diseases”.
I believe this probably holds true for all medical fields, where as we gain insights into the cause (etiology) of diseases we realize that one large disease is actually many orphan-size diseases all sharing some common phenotype. But it is important to talk about this, and it was important to hear this message in the large session, because it captures where much of the attention right now is going within the epilepsy space: to the rare diseases.
And this was particularly visible at AES 2023, where many of the pharma stands at the exhibit floor and pharma-sponsored sessions focused on rare epilepsy syndromes. As last year, this was largely focused on the most famous syndromes: Dravet syndrome, LGS and TSC; and this year also included CDD, the 4th rare epilepsy syndrome with an approved treatment, and the subject of an additional Phase 3 trial by UCB pharma.
Yet the poster session gave us a glimpse into the future, and I would like to highlight a poster from Biogen on KCNT1 and two from UCB on STXBP1 and SLC6A1. Although all preclinical, these posters are important because they illustrate how the large companies are now proactively working on the genetic epilepsy syndromes, not just watching the field (as they did at first) or buying the orphan drugs developed by others (more recently). This is an important step for the industry.
With large biotech and pharma now on board with genetic epilepsy syndromes, we are reaching a more mature stage in that transition from only working on seizures to also working on genetic causes. And this is happening without taking attention away from the larger seizure treatment space, where I would highlight the impressive results with XEN1101 so far in focal-onset seizures and potential also for generalized seizures. This is a very good scientific time for the epilepsy drug treatment space in general.
2 – RARE EPILEPSIES AS A TRANSITION POINT IN THERAPY DEVELOPMENT IN EPILEPSY
Are rare epilepsy syndromes the future of epilepsy treatment? Not at all. The syndromes are rather a natural step in the transition from symptomatic treatments to treatments that treat the cause of the disease.
I really liked a presentation by Dr Gemma Carvill from Northwestern about the history of epilepsy. She highlighted how most of the progress so far has been not on efficacy (we always hit that 30-35% of refractory patients) but on having better tolerability, and how more recent development focused on rare epilepsy syndromes, then on genetic treatments for these syndromes, and how the future direction is to use those technologies to treat epilepsy at large. When applied to the non-monogenetic epilepsies, the main approaches would be gene regulation approaches, moving up or down the expression of important targets, instead of activating or inhibiting proteins as small molecules often do.
Important advances to modulate gene expression in epilepsy are dCas9 approaches (for example Colasante 2020), the use of activity-dependent promoters (for example Qiu 2022), or the targeting of non-coding RNAs (reviewed in Stine 2023). And one of the best examples of going beyond the rare epilepsies is the AMT-260 gene therapy program from uniQure for Temporal Lobe Epilepsy. Gene therapy trials are not only for monogenetic epilepsy!
So genetic epilepsy syndromes are not the ultimate destination, but the first application of a new way of treating epilepsy, and this is becoming more clear as the epilepsy field evolves.
3 – PRESSURE IS HIGH, ALL SYNDROMES NEED BETTER TREATMENTS
Dravet syndrome is one of the epilepsy syndromes that has received the most attention from the industry. It has three drugs approved, soticlestat in phase 3 trials, and is the target of the most advanced clinical trial with a therapy designed to correct the cause of an epileptic syndrome: the ASO STK-001 from Stoke to correct the haploinsufficiency in SCN1A. This makes Dravet syndrome the “lucky syndrome”, and I have even seen grant proposals for Dravet turned down because it was considered “already done” by reviewers.
Yet at the Dravet syndrome roundtable we got to hear about 6-year old Anna and her journey with Dravet syndrome from her grandpa Ted Odlaug, who is the current president of the US Dravet Syndrome Foundation. During her short life, Anna has tried multitude of anti-epileptic drugs, including the three approved for Dravet syndrome, yet her epilepsy has gotten worse and worse and she currently has no days without seizures. She also has the cognitive development of a 2-year old, limited vocabulary, and her high seizure frequency including nocturnal seizures place her at very high risk for sudden death (SUDEP). Yet she got the “lucky syndrome”, the one with the most advances towards treating the symptoms and the cause.
This is something important to hear and to remember. All of what we have in epilepsy syndromes are some treatments approved to treat seizures, which work in SOME patients and that are tolerable by SOME patients. The treatments to correct the cause of these diseases are still in early development and still only accessible in the context of clinical trials. And this holds true even for the “lucky syndrome”. Most other syndromes are still behind in terms of therapy development.
So there is a big gap between the science, where we have cured the mice with SCN1A deficiency with several gene therapies and ASOs, and what patients in the real world like Anna have access to. Why is there such a progress gap between science and patient care? Part of it is time, treatments simply take years to get from the lab to the market, but we are also facing challenges bigger than time. And we are seeing these additional challenges as the first disease-targeting programs make their way to clinical trials and progress slows down. I discuss this in the next section. For this section my main message is that all syndromes need better treatments, and that none of them is yet “done”.
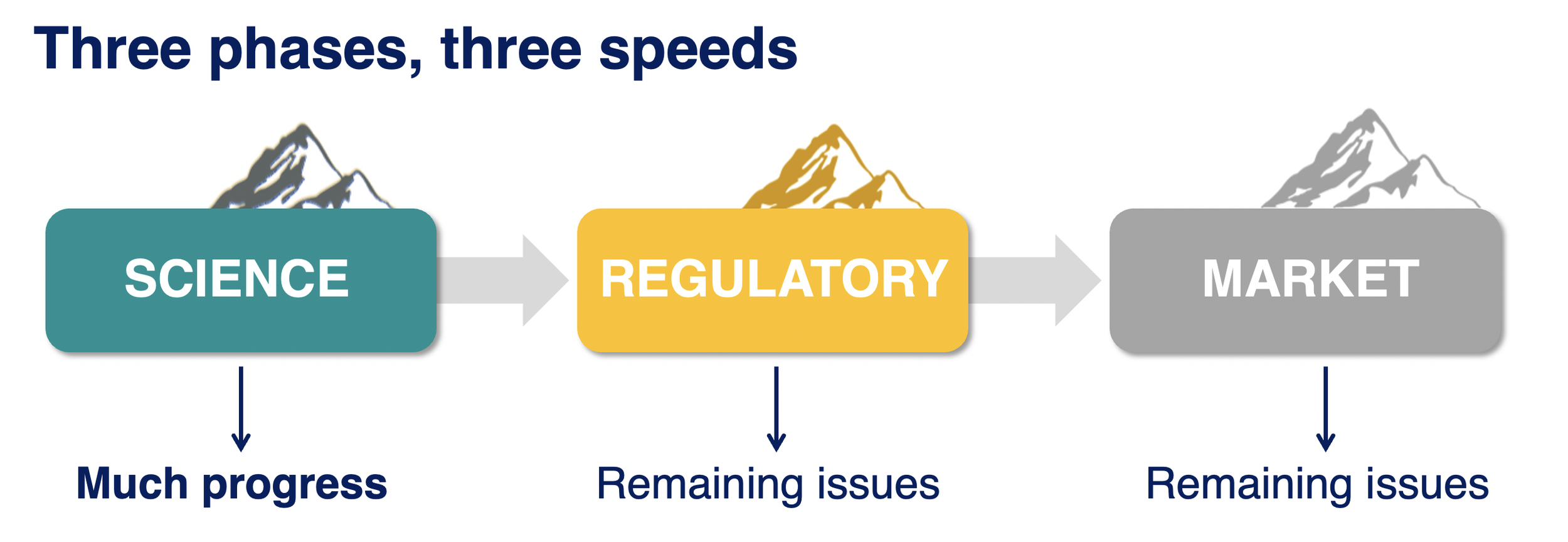
4 – RARE DISEASE THERAPY DEVELOPMENT HAS THREE MOUNTAINS TO CLIMB
Do you know the metaphor of a climber struggling to climb a mountain only to reach the summit and realize that there are other mountains just as high behind it? I believe this is where we are right now in developing disease-targeting treatments for genetic epilepsy syndromes, we are getting to the summit of that first mountain and looking at the next two. It turns out that getting gene-targeted treatment to trials was just the first mountain.
We had many discussions about these challenges during AES 2023. In fact, this was probably the most common topic across my many discussions with other attendees.
Mountain 1: Science.
Science has been advancing quite well. There are many preclinical programs with gene therapies and ASOs ongoing for a growing number of epilepsy syndromes, and we start having clinical trials. This year we have seen newer data from Stoke showing that with the highest dose of STK-001 they can have profound seizure reduction in patients with Dravet syndrome, and that even with moderate doses they can see improvements in non-seizure domains like communication. And Praxis has also started clinical trials with another ASO, PRAX-222, this one to reduce SCN2A in patients with gain-of-function mutations. This is in addition to Praxis’ small molecule trial for SCN2A and SCN8A gain-of-function DEEs.
We have also seen this year hopeful clinical trials results in Angelman syndrome (Ultragenyx) and Rett syndrome (Taysha) that make us all very hopeful that the neurodevelopmental field is getting closer to true disease-modifying treatments. So overall we start seeing the transition from preclinical ASO and gene therapies to clinical trials. This is just the first mountain.
Mountain 2: Regulatory.
This is a difficult one. We are still not sure how to design a clinical trial to show the difference between correcting the gene versus treating the seizures, and there are no precedents for a broader label to treat the disease (all we have are approvals “for the treatment of seizures in syndrome X”).
The patient community, clinicians and the companies developing treatments to target the cause of the epilepsy syndromes are working to solve this challenge by running observational clinical trials to validate non-seizure outcomes and endpoints.
The most advanced studies are the ones on Dravet syndrome by Stoke and Encoded, that have documented how, for example, the number of seizures in Dravet syndrome remains constant over time (up to 2 years!) as a group average. So while one patient might get better or worse, if you run a trial the number of seizures should balance out and remain stable. Many other symptoms are also stable or improve just a little, which will help us interpret open-label studies that may show improvements. And there are also other endpoint-enabling observational studies going on for CDD, SYNGAP and STXBP1 (at least) that I know.
We still don’t know which of these scales will be useful in clinical trials to show improvements beyond seizures, or how to convert the scales into specific endpoints, but in the meantime some of the companies with approved drugs to treat seizures in DEEs are leaning quite far into almost claiming efficacy in non-seizure outcomes by relying on caregiver surveys. And this is a development that worries me. We already have the problem in rare diseases of having to overrely on case studies due to so few placebo-controlled trials. And now we are adding the problem of companies with anti-seizure medications using caregiver surveys not reviewed by regulators to talk about improvements in behavior and cognition. This was particularly visible in 2023, and it almost creates two tracks with different burden of proof for non-seizure outcomes: one of post-approval caregiver surveys for small molecule anti-seizure drugs, and one of years of endpoint-enabling studies followed by year-long trials with not-yet-validated outcome measures for ASO and gene therapies. I’m not liking this development.
Mountain 3: Market.
A common challenge for rare diseases is not having enough patient numbers for an attractive return-on-investment. We have seen this addressed in rare epilepsy syndromes by developing the same molecule for a group of rare diseases. For example Epidiolex approved for Dravet, LGS and TSC; Fintepla approved for Dravet, LGS and in Phase 3 trials for CDD; and soticlestat in Phase 3 trials for Dravet and LGS. But running multiple parallel trials is expensive, so companies focus only on the largest syndromes.
A near-future regulatory innovation might be clustering syndromes into a broader label for drug development. This year we heard from the company Longobard, with a Phase 2 basket trials in DEEs, that they will consider discussing with regulators the potential to keep the combined DEE target population for Phase 3 trials and approval. I see regulatory challenges to do this but I also see it as the only way to get clinical trial data for many of the DEEs beyond the top 6 or so in terms of patient numbers, so I would love to see this happen.
The company Tevard is also considering a combination of DEEs, in their case because they are developing a gene therapy to help the cell skip premature stop codons caused by non-sense mutations, so their case is very similar to basket trials in the cancer field. In the case of Tevard, the target population would be genetic DEEs caused by non-sense mutations, and the common primary endpoint could be reduction in seizure frequency so one trial could test them all. Even a clinician working for Jazz told me that they had been asked by many clinicians to run studies with Epidiolex in groups of biologically-related DEEs (for example ion channels, or synaptopathies) so this definitely seems to be a conversation that we are hearing loudly in 2023 and that will likely turn into action.
5 – IMPRESSIVE PATIENT GROUPS
I have been working on rare epilepsies and supporting patient groups for 12 years now. And oh boy has that changed. Patient advocacy in 2023 is much more advanced and professional than how it was back in 2011.
The four epilepsy syndromes that have drugs approved all have very impressive patient-run foundations and patient groups behind them. See for example how the AES 2023 Extraordinary Contribution Award went to Mary Anne Meskis, Executive Director of the Dravet Syndrome Foundation. But they are no exception anymore. During AES 2023 I had a chance to interact with some of the patient groups for syndromes that still have no treatment approved (or advanced clinical trials) that were equally impressive.
Some of these deserve a shout out:
I attended the SYNGAP conference by the SynGAP Research Fund the day before AES. I wrote a separate summary about this conference, but this group is amazing in how they are tackling the key challenges to therapy development in their field, scientific/medical/industry community building, and patient mobilization including a very international footprint.
I caught the end of a meeting between the FamilieSCN2A Foundation and a biotech company, since I was next to meet with the company, and I was very impressed with their print slide-deck summarizing all of the resources and learnings from the SCN2A field. This included the entire list of mouse models and efforts to also validate outcome measures, for example in communication. This will help companies get up to speed with SCN2A quickly, and I’m pretty sure it will help start treatment programs by making it easier to choose this syndrome.
It is a tradition for me to sit down with Charlene and John from the STXBP1 Foundation every year at AES and compare notes about their field and the syndromes that I am working on. This year Charlene showed me one slide with the STXBP1 pipeline in 2019, having only one program for potential repurposing with nothing else on the horizon. And then the 2023 pipeline slide, with 11 programs in development including several gene therapies being developed by companies. Talk about progress!
CONCLUSSION: HAVE WE REACHED ESCAPE VELOCITY?
In science: yes. We are seeing a growing number of ASOs and gene therapies being developed for several of the neurodevelopmental syndromes with epilepsy. Transition to clinical trials is still a bit slow but the progress across many diseases is notable.
In regulatory path and market conditions: no. We are still not sure how to design a clinical trial to show the difference between correcting the gene versus treating the seizures, there are no precedents for a label that goes beyond seizures, and the market is still a limitation, leading to companies always choosing the largest syndromes and neglecting the other hundred or more.
We are seeing some development to address these challenges. Some are good developments, like considering how to get several syndromes into a single clinical program and approval. Some are (as I see them) bad developments, like companies promoting cognitive and behavioral efficacy for their drugs using a language identical to the one they use for seizure efficacy, which is the only one approved and on label.
Another development is the arrival of gene therapies to non-genetic epilepsy trials, which is very promising. Also exciting is to see big bio and big pharma proactively working on the genetic epilepsy syndromes, not just watching the field or buying the orphan drugs developed by others. And we are also now getting the results of the observational studies that are being carried out for several DEEs to identify endpoints for more complex clinical trials. So overall this was a good year.
For 2024 my hope is to:
see some answers to the regulatory challenges, with learnings from Stoke and also from other companies in the neurodevelopmental (non-epilepsy) field.
see more clinical trials started with treatments targeting the cause of the disease (potentially from the group of SLC6A1, SCN2A Loss-of-function, and CDD?)
see more mouse proof-of-concept data with treatments targeting the cause of the disease. We have many ideas and in vitro data, but not enough clinical candidates yet.
And I hope to see that in Los Angeles, for AES 2024.
Ana Mingorance, PhD
Disclaimer: These are my own impressions from the presentations and topics that I was most interested in. I write these texts with the parents of individuals with rare epilepsies in mind, so excuse also my lack of technical accuracy in parts.
REPASO DEL FORO CDKL5 2023
La novena edición del Foro CDKL5 tuvo lugar en Boston, los días 6 y 7 de noviembre. El Foro es una reunión anual que organiza la Fundación Loulou y en la que científicos y miembros de la industria farmacéutica se reúnen con representantes de la comunidad de pacientes para repasar los últimos avances en el campo.
Este es un repaso para los grupos de pacientes de las principales novedades del Foro CDKL5 2023.
FORO 2023
Hace ya nueve años que la Fundación Loulou organiza una reunión anual, el Foro CDKL5, donde los científicos de academia y de industria trabajando en el síndrome de deficiencia en CDKL5 (CDD), junto con representantes de los grupos de pacientes, se reúnen para compartir las últimas novedades y avanzar hacia tratamientos y una cura. Tenéis el resumen del año pasado aquí.
La edición de 2023 tuvo lugar en Londres los días 6 y 7 de noviembre, y volverá a Boston en 2024. Cada año digo que este ha sido el mejor Foro, pero es que es verdad. Tengo 47 páginas de notas por toda la información nueva y todos los momentos importantes que nos dio el Foro. Así que voy a intentar resumir las conclusiones principales de la edición de este año, no incluyendo todas las presentaciones sino centrándome en los temas principales y los progresos que vimos, que son muchos.
1. SEGUIMOS DESCUBRIENDO COSAS SOBRE CDD
El Foro siempre arranca y cierra con la voz de la comunidad de pacientes. Este año fue Cristina, de la Asociación de Afectados CDKL5, quién abrió el Foro con un mensaje muy poderoso. Nos explicó que cuando les llegó el diagnóstico de su hija, aprendieron que “viviría una vida a medio camino entre incierta y horrible”. Y que por eso, para allá, la investigación es la esperanza.
Y cada año la investigación nos sigue enseñando cosas nuevas sobre CDD que tienen importantes aplicaciones de cada a entender la enfermedad o a posibles tratamientos. Aquí os cuento algunas, y mirad también la sección 6 de este resumen.
La Dra Lauren Orefice de Boston nos enseñó como el rechazo a ciertas texturas en CDD es consecuencia de cambios sensoriales, no de comportamiento. Los ratones con CDD tienen las neuronas sensoriales demasiado sensibles a las texturas rugosas, reaccionan demasiado fuerte, y por eso suelen rechazar esas texturas. Y es muy posible que lo mismo pase en las personas con CDD que insisten en comer texturas específicas.
Varios proyectos de varios laboratorios coinciden en ver cambios de conectividad funcional en CDD, pero no cambios estructurales. O sea que las neuronas están en el sitio correcto y en general el cerebro está bien y bien conectado, pero algunas neuronas disparan juntas más de lo que deberían, y otras no disparan juntas tanto como deberían (eso es la conectividad funcional). Y para mi esto es muy prometedor de cara a tratamientos porque cuando normalizamos la actividad neuronal podemos obtener cambios en este tipo de conectividad funcional. Lo que sería mucho más difícil de arreglar es cambiar una neurona de sitio.
Sir Adrian Bird nos dio una de las ponencias estrella. Se hizo famoso porque fue el primer científico que demostró que el síndrome de Rett es reversible en ratones, y n os explicaba que en Rett (gen MECP2) tener poco o tener mucho es problemático, con lo que todas las terapias génicas en desarrollo para Rett (que es por falta de MECP2) llevan mecanismos de freno para no llegar a causar sobreexpresión (demasiado MECP2). Pues una de las sorpresas de este año nos vino de la Dra Sharyl Fyffe-Maricich de Ultragenyx, que nos demostró algo que sospechábamos desde hace algunos años: el cuerpo ya pone freno a cuanto CDKL5 puedes producir y no te deja hacer sobreexpresión. Nos enseñó datos con ratones y con primates, donde da igual cuanta terapia génica les des, las neuronas se comen el exceso de CDKL5. Y eso es buena noticia porque las terapias génicas de CDKL5 serán más fáciles al no tener que incorporarles ningún mecanismo artificial de freno.
Rechazando texturas y aprendiendo de Rett
2. ELIMINANDO BARRERAS PARA AVANZAR EL DIAGNÓSTICO Y LA INVESTIGACIÓN
El Foro 2023 nos trajo muchos ejemplos de generación de modelos de forma colaborativa, y quiero destacar un progreso en torno a diagnóstico y uno sobre modelos de epilepsia.
Un diagnostico genético no siempre es fácil de interpretar. Por ejemplo, si vuestro hijo o hija tiene una mutación en el gen CDKL5 que le rompe el gen, es fácil de interpretar en un test genético. Pero si tiene las de una letra cambiada por otra… esas son difíciles de interpretar y acabamos con un VUS (de las siglas en inglés para “variante de significado incierto”) y con un diagnóstico sin confirmar. Un equipo de la Telethon en Italia está haciendo en el laboratorio todas las mutaciones de cambio de letra posibles en CDKL5, probándolas en células, y van a poner todos los resultados en una base de datos. De ese modo, cualquier laboratorio genético encontrará respuesta para cualquier cambio de letra que vean en un niño. No más VUS en CDKL5. Un proyecto super chulo.
Y otro progreso enrome en generación de modelos de investigación que vimos este año es que por fin tenemos buenos modelos de epilepsia! La Dra Liz Buttermore nos enseñó un modelo in vitro, a partir de neuronas de pacientes en placas de cultivo, donde las neuronas de pacientes tienen una hiperactividad clara (disparan mucho). Y como científica que trabajó en la industria farmacéutica os puedo decir que este es el tipo de modelo que me hubiera gustado tener para probar fármacos. Luego está si recordáis el Dr Muotri de California que hace organoides (bolitas de neuronas) a partir de células de pacientes, que son como bolitas de corteza cerebral. Pues este año la Dra Rebeca Blanch, de su laboratorio, nos enseñó como las van a hacer tálamo-corticales para que puedan tener ese circuito tan importante para la epilepsia. Y el Profesor Peter Kind nos enseñó cómo su laboratorio ha encontrado el foco epiléptico en el cerebro de ratas con CDD, y ahora pueden estimularlo directamente para provocarles crisis cada vez que quieren. Este es un modelo fantástico para estudiar tratamientos. Sin duda en este año hemos tenido un avance tremendo en esta área.
Deshaciendo VUS y epilepsia en ratas con CDD
3. LOS “4 APROBADOS” Y LOS FÁRMACOS EN DESARROLLO
En 2022 se aprobó ganaxolona en Estados Unidos y se convirtió en el primer fármaco aprobado para CDD. Luego en 2023 nos ha llegado la aprobación Europea.
Y para que veáis lo excepcional y lo difícil que es tener un fármaco aprobado para un síndrome con epilepsia, os voy a repetir lo que dije durante mi charla en la sesión sorbe tratamientos: hay más de 300 síndromes con epilepsia, y solo 4 tienen un tratamiento aprobado. Estamos hablando de Lennox-Gastaut, esclerosis tuberosa, síndrome de Dravet y CDD. Ya está, solo esos 4.
Y una empresa de biotecnología empezó a llamarlos este año “los 4 aprobados”, por ser la excepción. Y para las empresas de desarrollo de tratamientos, estos 4 aprobados son más fáciles que cualquier otro, porque ya existe un precedente y por tanto respuestas a muchas preguntas de cómo llegar a la meta en esa enfermedad, y eso los hace más atractivos para tener más tratamientos. Por eso el valor de ganaxolona va más allá que el valor como medicamento.
Hemos pasado de haber tenido cero ensayos clínicos, a tener 8 en 8 años, y formar parte de “los 4 aprobados”. Para ser una enfermedad relativamente nueva, hemos empezado muy fuerte.
Y ahora la pregunta que nos queda es “y después de ganaxolona qué”. Y en el Foro vimos varias presentaciones de las terapias que vienen después, y que resume en esta sección y las tres siguientes.
Marinus anunciaba en el Foro que acaban de firmar un Programa de Acceso Global para llevar ganaxolona a los países donde no está disponible comercialmente (o en proceso de estar disponible como sería el caso de Europa). Su web tiene un correo de contacto para que los médicos puedan informarse. También nos enseñaron datos de que ganaxolona mantiene eficacia durante al menos 2 años.
UCB Pharma estuvo representada por dos personas, incluida la directora médica que dirige todos los ensayos clínicos, y nos explicaban que el ensayo clínico de fenfluramina para CDD en fase 3 está en marcha en Asia, Europa y Estados Unidos, con una web muy fácil de recordar: cddstudy.com
También repasamos varios fármacos en desarrollo para otras epilepsias o para otras enfermedades de neurodesarrollo, y que podrían potencialmente incorporarse a la lista de ensayos para CDD. Esos incluyen otros fármacos que actúan sobre el GABA, los activadores de KCC2, los inhibidores de Cav2.3 (os cuento más en la sección 6) y algunos tratamientos dirigidos a la flora intestinal que me resultan muy interesantes.
La conclusión de esta sesión es que ganaxolona fue solo el principio y que hay muchas opciones por detrás.
Presentaciones de empresas con terapias en desarrollo
4. CLINICAL TRIAL READINESS: CÓMO LA COMUNIDAD DE PACIENTES ESTÁ HACIENDO POSIBLES LAS TERAPIAS DEL FUTURO
Al terminal el Foro nos han llegado correos de gene de empresas que vinieron a Londres y todos destacan esta sesión como muy importante. Como nos escribió uno literalmente “esto va a atraer la participación de más empresas”.
Se trata de la sesión sobre biomarcadores y escalas clínicas, que responden la pregunta de “cómo vamos a medir en ensayos todo lo que no es epilepsia”. Y es particularmente crítico para los ensayos con terapias génicas, y en los últimos 12 meses hemos tenido muchos avances importantes.
Los biomarcadores son formas de medir cambios en el cerebro sin tener que hacer una biopsia (por razones obvias algo no viable para el cerebro). Un tipo de biomarcadores son los que podemos medir en sangre (los niveles de azúcar serían un biomarcador de diabetes) y el Dr Massi Bianchi de Ulysses nos enseñó datos muy prometedores de poder medir plasticidad neuronal en un análisis de sangre, si nos fijamos en dos marcadores en concreto. Otro tipo de biomarcador son los de técnicas de imagen cerebral, y el Dr Eric Marsh de Filadelfia nos enseñaba también datos de cómo hay algunas señales del electroencefalograma que correlacionan con la severidad de la enfermedad en CDD. Son dos tipos de marcadores que muy posiblemente se usarán en ensayos clínicos.
Luego el Dr Marsh nos compartió los resultados interinos de un estudio americano para desarrollar nuevas escalas clínicas para CDD. Ya han completado la primera parte y ahora las están refinando y confirmando, y tiene muy buena pinta. Y quiero destacar un detalle: este es un estudio observacional con más de 100 participantes, y 100 es justamente el número de pacientes de un ensayo de Fase 3 en CDD, así enhorabuena a la comunidad de pacientes por movilizarse de tal manera para hacer este estudio y los de los biomarcadores sean posibles.
Y por último el Dr Xavier Liogier, de la Loulou Foundation nos actualizó sobre el estudio CANDID, que es parte de un consorcio con varias empresas que están desarrollando tratamientos para CDD: El estudio CANDID busca validar en CDD escalas que ya se han usado en ensayos para otras enfermedades. Hace un año, Xavier nos contaba que el primer paciente ya había entrado al estudio. Ahora 12 meses después nos decía que ya tienen más de 100 visitas programadas con lo que cierran reclutamiento, y nos enseñó ya muchos de los datos que tienen viendo cómo estas escalas se aplican a CDD. El gráfico de reclutamiento al final parecía exponencial, y de nuevo muestra la tremenda movilización de la comunidad de pacientes para hacer este estudio posible.
El Dr Billy Dunn, que era el director de neurología de la FDA y ahora es Asesor Senior de la Fundación Loulou, fue moderador de esta sesión y alabó el espíritu de colaboración de los participantes por enseñarnos datos mientras los estudios están aún en curso, y destacó también el valor de estar construyendo “un menú del que elegir” para hacer posible diseñar ensayos complejos en CDD.
Proyecto de biomarcadores de Ulysses y curva de reclutamiento exponencial de CANDID
5. HACER UNA TERAPIA GÉNICA ES COMO CONSTRUIR UNA CASA
Hace un año, en el Foro 2022, pensábamos que en este Foro tendríamos la noticia de que una de las terapias génica había obtenido permiso para empezar ensayos. Pero todavía no hemos llegado a esa noticia.
Y hablando con algunos de los padres en el Foro sobre cómo explicar a gente que no es científica las implicaciones de que haya retrasos en este tipo de proyecto complejo, lo mejor que encontramos es el ejemplo construir una casa que compras sobre el plano. Si no lo habéis vivido directamente seguro que conocéis a alguien a quien le ha pasado: firmas sobre plano, el constructor te dice el plazo en el que te podrán dar las llaves, y al final raramente es en esa fecha. Y es que hay demasiadas etapas que pueden añadir retrasos, pero eso no quiere decir que un retraso indique que hay un problema con la casa o que no te la llegarán a entregar. Puede que hacer los cimientos se retrase por un invierno largo, el aislamiento haya que retocarlo, la estructura exterior de la casa nos de alguna sorpresa… añade a eso todas las instalaciones, los acabados exteriores y los interiores… hay demasiados pasos que pueden alterar esas predicciones de fechas.
Hacer una terapia génica es como construir una casa. Los expertos en biotecnología saben cómo hacer estas terapias, ya lo han hecho antes, pero aún así tienen que hacer la tuya desde el principio y hay muchos pasos complejos incluidas las inspecciones (igual que en una casa!) que acaban moviendo los plazos pero que son parte normal de hacer una terapia génica.
Lo que sabemos hoy es que tanto la terapia génica de Ultragenyx como la que está haciendo la Fundación Loulou con la Universidad de Pensilvania ya han hablado con las agencias reguladoras en 2023, y que ambas esperan obtener la aprobación para ensayos en 2024. Así que el proceso ha sido bueno, aunque no tan rápido como queríamos. Y Sharyl de Ultragenyx fue muy clara en este Foro: no se han encontrado ninguna señal adversa de seguridad con la terapia génica de CDD en ninguno de sus estudios con animales realizados hasta la fecha, incluyendo los estudios de seguridad en primates. Es simplemente un caso de tener que hace run poso más de trabajo para pasar la inspección de la casa.
En resumen al final del año pasado nos dijeron “creo que te daremos las llaves de la casa el año que viene” y no ha sido así, pero no hay ningún problema con la casa. Es que es de verdad muy complicado atinar con las predicciones en proyectos tan complejos.
Y en el Foro 2023 repasamos también los progresos en desarrollar terapias génicas con modalidades nuevas, de las que no hay aún precedentes aprobados para otras enfermedades. En concreto vimos un update del Dr Kyle Fink, que está desarrollando una terapia génica basada en CRISPR para abrir la segunda copia del gen CDKL5 en niñas (la del segundo cromosoma X), y de Kelcee Everette que trabaja en el laboratorio del Dr David Liu y que también está desarrollando una terapia génica basada en CRISPR, en este caso para arreglar la letra mutada o poner la letra que falte en el gen. En ambos casos se centran en aumentar la eficacia de corrección del gen, aumentar la selectividad (que no cambie genes que no tocan) y hacer los CRISPR más pequeños porque todavía no caben en los virus que se usan para terapia génica. Estos proyectos son como hacer casas con materiales nuevos, que no se han usado todavía nunca, y que de momento son demasiado grandes para la casa. Con lo que los plazos para su llegada a ensayos son impredecibles, pero el progreso es muy tangible. Y a mi me parece aún increíble que CDD sea de las primeras enfermedades a las que se están aplicando estas terapias génicas del futuro.
Varias terapias génicas en desarrollo
6. DOS SORPRESAS QUE HAN ABIERTO DOS PUERTAS
No todo está tardando más de lo que pensábamos. Hay cosas que están pasado más rápido de lo que podríamos imaginar, y vimos dos ejemplos notables de esto en el Foro.
Aprendiendo de la evolución
El Dr Ibo Galindo del Príncipe Felipe nos presentó un análisis evolutivo fascinante del gen CDKL5 desde la primera célula de la evolución y pasando por el árbol entero de la vida. Resulta que las plantas tuvieron en origen un gen CDKL, pero lo perdieron (¡tienen deficiencia en CDKL5!), que el gen CDKL5 original fue el 5, pero que luego se duplicó varias veces dando lugar a las 5 versiones. Y también aprendimos que mientras que las cinco formas CDKL1-5 tienen funciones importantes de regulación del esqueleto celular (el citoesqueleto), el gen CDKL5 parece haber adquirido funciones nuevas en vertebrados. Había dos conclusiones importantes: (1) es posible que haya redundancia funcional entre alguno de estos genes, y (2) CDKL5 tiene una cola especial en vertebrados que indica que tiene una función que no es de regular citoesqueleto.
Y estas conclusiones nos pusieron un marco de fondo perfecto, para dos proyectos que vienen del laboratorio de la Dra Sila Ultanir del Crick Institute y que os cuento más abajo. El laboratio de Sila se llevó el premio al Laboratorio del Año en el Foro 2023, pero a mi me gusta llamarlo el laboratorio de la década por el impacto que tienen en la investigación en CDD.
1. Redundancia funcional. Encontramos el parálogo. Encontramos avenida terapéutica.
En biología, un gen paralógo es el que nace por duplicación de otro, y ahora se parecen tanto que posiblemente compartan algunas funciones. Esto es lo que pasó en la atrofia muscular espinal, esa enfermedad neuromuscular letal en bebés que los científicos solucionaron porque encontraron un gen SMN2 (¡un parálogo!) que podía compensar por la falta de SMN1.
El laboratorio de Sila se dio cuenta de que una de las proteínas de citoesqueleto más famosas controladas por CDKL5, que se llama EB2, no estaba controlada solo por CDKL5 en neuronas, había otra kinasa que también la regulaba. ¡Y buscando quien era encontraron que era CDKL2! Y ahora están buscando la posibilidad de aumentar la expresión de CDKL2 en CDD para compensar por la falta de CDKL5. Y hay una posibilidad de hacerlo con ASOs. Una de las ponencias invitadas fue por el Dr Stanley Crooke, que es el fundador de la empresa Ionis (la que hizo el primer oligonucleótido antisentido o ASO para tratar la atrofia muscular espinal), y que luego a fundado una ONG llamada N-Lorem para hacer ASOs específicamente para gente que tiene enfermedades genéticas “nano-raras”. Y dijo en su charla que “CDKL2 tiene los elementos arquitecturales genéticos necesarios para hacerle un buen candidate a aumentar su expresión usando ASOs”. Y se eso sabe mucho este hombre.
Ha sido muy inesperado enterarnos de que quizás podamos seguir los pasos de la atrofia muscular espinal al encontrar un segundo gen que pueda compensar con el primero (al menos en neuronas, porque parece que en otros órganos son otros CDKL los que compensan). Ha sido un descubrimiento y comienzo de un proyecto terapéutico que nos ha llegado super-rápido.
2. Predicción que tiene dianas que no son de citoesqueleto. Encontramos la diana. Encontramos avenida terapéutica.
La Dra Marisol Sampedro, que trabaja con Sila, nos presentó el año pasado su descubrimiento de que CDD podría ser parcialmente una canalopatía porque encontró que el canal de calcio Cav2.3 era una diana clave de CDKL5. Cuando falta CDKL5, el canal Cav2.3 no está fosforilado (el interruptor que le pone CDKL) y se queda demasiado abierto, y la neurona se queda demasiado activa. Así que lo que necesitaríamos son inhibidores de este canal, y esto lo repasamos en el resumen del año pasado donde lo llamé un descubrimiento muy importante.
Pues un año después teníamos en el Foro una empresa nueva llamada Lario Therapeutics contándonos que están desarrollando fármacos inhibidores de Cav2.3 para CDD y para otro síndrome causado por mutaciones de ganancia de función en ese gen (también tienen Cav2.3 con demasiada actividad). El director científico de Lario nos explicó que están terminando la optimización química de los compuestos y que pasarán a los estudios de seguridad en animales previos a ensayos clínicos. Estamos hablando de una medicina de precisión para CDD y esto de verdad que nos ha llegado mucho más rápido de lo que podríamos haber imaginado.
RESUMEN: MOMENTUM
Este año aprendimos que la primera terapia génica está tardando más de lo que pensábamos, pero muchas otras cosas están pasando más rápido que lo que hubiéramos pensado, como esas dos nuevas oportunidades terapéuticas (CDKL2 y Cav2.3) que eran inimaginables hace tan solo dos años. También vimos por fin los frutos de varios esfuerzos de varios años de duración, como los dos estudios observacionales que han reclutado más pacientes que un ensayo de Fase 3, o tener por fin modelos de epilepsia in vitro y en ratas que permiten el tipo de experimento que necesitan las empresas farmacéuticas. Así que la impresión que yo me llevo es de que la velocidad de progreso se está acelerando, un poco como este gráfico de reclutamiento del estudio CANDID, y que hemos llegado a un momentum importante.
Y esa es justamente la palabra que Stan Crooke usó cuando se subió al podio y nos dijo “el momentum que tenéis ahora mismo en investigación en CDD es palpable”. Por eso creo que es buena palabra para capturar lo que vimos este año (aunque no la usemos mucho en español, es una especie de inercia inparable).
Y ese momentum se apoya sobre dos pilares que también se mencionaron mucho durante este Foro: la determinación y el espíritu de colaboración.
La voz de las familias en el Foro 2023
Así que os dejo con las frases de tres de los padres/madres que hablaron en el Foro 2023 y que capturan estos mensajes:
Cristina, de la Asociación de Afectados CDKL5, que nos recordó que “las personas con CDD son increíbles y se merecen lo mejor”
Majid, de la Fundación Loulou, que reconocía el camino que queda por recorrer explicando que “hemos avanzado más rápido y más lejos de que lo hubiera imaginado, gracias a todos vosotros. Pero al mismo tiempo, nunca es tan rápido ni tan lejos como querríamos las familias” y pedía a la audiencia que se volvieran a comprometer a curar la enfermedad con aún más pasión, más motivación y más determinación
Y por último Heike, en representación de la Alianza Internacional, nos hablaba de la personalidad de su hija, de los esfuerzos que los diferentes miembros de la Alianza están llevando a cabo, y cómo “estamos en esto juntos”.
Ya se que digo lo mismo todos los años, pero el de este año fue el mejor Foro que hemos tenido.
Espero que os haya gustado el resumen. Ya me diréis lo que os parece en los comentarios.
Ana Mingorance, PhD
Nota: este texto captura mis impresiones de las presentaciones del Foro que más me interesaron, no es un texto oficial del congreso emitido por la Fundación Loulou. Escribo estos resúmenes para los padres de personas con CDD, así que a veces me tomo ciertas licencias a la hora de explicar las partes mas técnicas.
MAIN LESSONS FROM THE 2023 CDKL5 FORUM
For the past nine years the Loulou Foundation hosts an annual meeting where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances.
Here are the main news and take-home messages from the 2023 CDKL5 Forum that took place in November 6-7 2023
2023 CDKL5 Forum
For the past nine years, the Loulou Foundation has hosted an annual meeting, the CDKL5 Forum, where scientists and drug developers working on CDKL5 Deficiency Disorder (CDD), together with representatives from patient organizations, meet to discuss the latest developments in the field and to advance towards treatments and cures. You can find summaries from the past few meetings here: 2018, 2019, 2020, 2021 and 2022.
The 2023 CDKL5 Forum edition took place November 6-7 in London, UK, and will return to Boston next year for the 2024 edition. Each year I say that this was the best Forum so far, but it is true. I wrote 47 pages with notes. There was so much good information and so many important moments! I will try to summarize the main take-home messages from this year’s Forum including the scientific pre-forum meeting. This won’t cover all the presentations, but rather focus on the main themes that we saw and the main progresses – which are many.
1. USEFUL NEW INSIGHTS INTO CDD
The Forum always opens and closes with the words from the patient community. This year, Cristina, from CDKL5 Spain, opened the conference with some powerful words. She explained that when they received their daughter’s diagnosis, they also learnt that their daughter “would live a life somewhere between uncertain and horrible”.
That’s why for her, research means hope.
And every year we keep learning new things about CDD that have important implications to understanding the disease and thinking about treatments. Here are 3 of those, and check out also point #6 in this summary.
Dr Lauren Orefice from MGH and Harvard showed us that food texture aversion in CDD happens because of sensory changes, rather than behavioral reasons. Mice with CDD have sensory neurons that are hyper-reactive to rough texture, so no wonder they don’t like it. This is probably the same for people with CDD who might have difficulties with specific food textures.
Several research projects keep finding functional changes in brains with CDD, rather than structural changes (Dr Tanaka, Dr Higley, Dr Fagiolini and others). This means that neurons are in the right place, and overall the brain is all well shaped and connected, but some neurons are firing together more than they should while others are not firing together as much as they should. To me, this is promising for treatments because normalizing neuronal activity can lead to plastic changes in this type of neuronal functional connectivity. What would be much harder is to try to correct having neurons in the wrong place.
We had a wonderful lecture by Sir Adrian Bird, who became famous after showing that Rett syndrome could be reversible in mice. He explained to us how in Rett syndrome too little or too much MECP2 are both bad options, so the gene therapies in development for Rett syndrome (too little MECP2) all incorporate control mechanisms to prevent running into overexpression MECP2. A big surprise from this Forum was Dr Sharyl Fyffe-Maricich from Ultragenyx confirming with data in mice and primates what we had suspected for several years: the body puts a limit to how much you can express CDKL5 and it will not let you get into overexpression (too much) of CDKL5. The neurons eat all of the excess away, no matter how much gene therapy you give them. This is very encouraging, because it makes gene therapies for CDKL5 easier since we don’t need to add that “break”.
Food texture aversion and learning from the Rett syndrome field
2. SOLVING KEY PROBLEMS TO ADVANCE DIAGNOSIS AND RESEARCH
The 2023 Forum showed even more collaborative reagent and model generation than previous years, and in particular I would like to highlight advances around diagnosis and seizure models.
Genetic testing is not always easy to interpret. For example, if your child has a mutation in the CDKL5 gene that breaks the gene, then it is easy to interpret in a genetic test. But what happens when the mutation changes a letter by another one? This one is hard, and we end up with VUS (variant of unknown significance) and an uncertain diagnosis. A team from the Telethon Institute of Genetics and Medicine (TIGEM) is making in the lab all of the possible letter changes in the CDKL5 kinase domain and will check all of their functions in cells and then put the results in a database. That way, genetic labs will always have an answer for any letter change that they find. No more VUS for CDKL5, what a cool project.
And another big research tool progress from this year was that lots of seizure-relevant models are finally available! We had Dr Liz Buttermore show us a beautiful plate assay with cells from patients with a very nice hyperexcitability signal in patient-derived neurons. As a former pharma scientist, I know this is the type of assay that I would like to use. If you remember from other years, Dr Muotri from UCSD had a great in vitro CDD model using organoids from brain cortex. This year Dr Rebeca Blanch from his lab showed us that they are now making thalamo-cortical organoids, to try to better recreate one the main epilepsy circuits. Very cool! And Prof Peter Kind showed us how his lab has identified an epilepsy hotspot in CDD rat brains and they are able to stimulate it to induce seizures whenever we want. That’s such as great epilepsy model! This year we had incredible progress in this area.
VUS databases and CDD rat seizure model
3. “THE APPROVED 4”, AND TREATMENT PIPELINE TODAY
CDD had the first drug approved in the US (2022) and Europe (2023).
To show you just how special and how hard it is to have a drug approved for a rare epilepsy syndrome, I will repeat here what I said in my talk during the treatment pipeline session: there are more than 300 epileptic syndromes, and there are only 4 epilepsy syndromes that have any drug approved. These are Tuberous Sclerosis Complex (TSC), Lennox-Gastaut Syndrome (LGS), Dravet syndrome, and CDD. That’s it, just 4.
A pharma company recently started referring to these as “the approved 4” because it is so exceptional for an epilepsy syndrome to have a drug that has made it all the way to the finish line. For companies looking to develop treatments, these approved 4 are going to be easier and therefore more attractive than the others, because there is already a precedent and therefore many answers to how to get a drug to approval for them. So the value of ganaxolone goes beyond the value of the medicine.
We have gone from zero to 8 clinical trials in 8 years and have become part of the approved 4. This is a very strong beginning.
Now the question that remains is “what’s next”. At the Forum we had several presentations for the therapies that come next and many of the efforts to support them, which I summarize here and in the next three sections.
Marinus Therapeutics announced at the Forum that they have now signed a Global Access Program to help get ganaxolone to people with CDD in countries where it is not commercially available (or in process of becoming available, like is the case for Europe). Their website has a contact email for doctors to reach out to learn more about access. They also showed nice data of sustained seizure efficacy with ganaxolone over 2 full years.
UCB Pharma was represented by two speakers, including their CMO, who explained that UCB spends 30% of their revenue in R&D – this is a lot for the industry. And as you know some of that went to acquire Zogenix and their drug fenfluramine, which is now in Phase 3 trials for CDD. The trial has a website with an easy to remember name: cddstudy.com and you can see there all the countries where the trial is available in Asia, Europe and North America.
We also reviewed several drugs in development for other epilepsies or other neurodevelopmental disorders and that could potentially join the CDD pipeline in the coming years. These included other GABA drugs, KCC2 activators, Cav2.3 inhibitors (which we review in section 6) and some treatments that target the gut microbiome and that I find very interesting.
The conclusion for the session on the current CDD pipeline was that ganaxolone is just the beginning and that are multiple options coming behind it.
CDD therapeutic pipeline presentations
4. CLINICAL TRIAL READINESS: HOW THE PATIENT COMMUNITY IS MAKING THE FUTURE THERAPIES POSSIBLE
After the Forum we got emails from pharma and biotech industry professionals that attended the Forum and they all mentioned this session as very impactful. As one literally wrote, “this will attract more industry participation”.
This session was about biomarkers and outcome measures, which is “what to measure in trials that will look at more than seizures”. This is particularly critical for the future gene therapy trials, and the last 12 months have seen tremendous progress.
Biomarkers are way to see changes in the brain without directly taking a biopsy. One type of biomarkers are the ones we can measure in blood (glucose levels are a biomarker for diabetes), and Dr Massi Bianchi from Ulysses showed us very promising early data for measuring neuronal plasticity in a blood test by looking at two key markers. Another type of biomarker is using brain imaging, and Dr Eric Marsh from CHOP showed us data from a large collaboration where they can correlate EEG signals with disease severity in CDD patients. Both of these types of biomarker are very likely to be used in future clinical trials.
Dr Marsh also updated us on the development of novel clinical scales to measure CDD symptoms beyond seizures run by the ICCRN network. They have completed a first part and they are now tweaking and confirming the new scales, which are looking really promising. And one important bit to highlight: this is a study with over 100 patients, and 100 patients is the size of a Phase 3 trial in CDD so kudos to the patient community for mobilizing to make this work and the biomarker studies possible.
And then Dr Xavier Liogier, from the Loulou Foundation, updated us on the progress around the CANDID study which is run in collaboration with a consortium of companies developing CDD treatments. This study evaluates well-known clinical scales that have already been used in clinical trials but for other diseases, to see how they may apply to CDD. Last year Xavier told us about the first patient having been enrolled. Twelve months later he told us that we will get more than 100 participants by the end of this year and showed us a lot of data for how these scales are able to capture different CDD symptoms. The recruitment graph looked exponential, again showing the tremendous mobilization of the patient community to make the CANDID study possible.
Dr Billy Dunn, former Director of the Office of Neuroscience at FDA and current Senior Advisor to the Loulou Foundation, moderated this session and praised all presenters for the spirit of collaboration in sharing all these data while still in progress, and highlighted the value of building all these biomarkers and scales as a “menu to choose from” to enable complex trials in CDD.
Ulysses biomarker project and exponential CANDID recruitment curve
5. MAKING A GENE THERAPY IS LIKE BUILDING A HOUSE
One year ago, at the 2022 Forum, we through that by the end of 2023 we would have the announcement that one of the gene therapies for CDD had received green light for a trial. We didn’t get this news.
And discussing with some parents at the Forum about the best way to explain to non-scientists the implications of delays in very complex process projects, we came up with the idea of how this is very much like building a house from scratch. You approve the initial design, and the constructor gives you a timeline for when you will get the keys. Then it rarely ever happens as planned. And that is because there are too many steps that can end up taking a bit longer, but this is not a sign that there is a fundamental problem with the house or that you will never get the keys. It may be that pouring the foundation is delayed due to a long winter, the insulation had to be redone, building the skeleton of the house can also unlock some surprise… add to that installations, exterior finishes, then all of the interior work… there are just many steps that impact timeline predictions.
Making a gene therapy is not unlike building a house. The biotech experts know how to make gene therapies, but they still have to adjust each step to our particular gene therapies and there are so many complex steps including regulatory reviews (like the different house inspections!) that timelines end up shifting but that is just a normal part of making a gene therapy.
What we know today is that the gene therapy from Ultragenyx and the one that the Loulou Foundation is developing with UPenn have indeed both already been presented to regulators in 2023, and are both now hoping to get clinical trial approval in 2024. So the progress has been good, just not as fast as we all hoped for. And Sharyl from Ultragenyx was very clear in her presentation this year: they have not found any adverse safety signals with the CDD gene therapy to date in any of their studies in animals including primates. This is a case of having to complete some more extra work to pass the house inspection.
Bottomline: at the end of last year we were told “I think I can give you the keys to the house by the end of next year” and that didn’t happen yet, but there is no problem with the house. It is just really hard to predict the exact timelines in very complex projects.
At the 2023 Forum we also reviewed the progress using new gene therapy modalities that haven’t been yet approved for any human disease so there are no precedents. In particular we had an update from Dr Kyle Fink from UC Davis who has been developing a CRISPR-like approach to reactivate the second copy of CDKL5 gene in each cell in females (from the second X chromosome), and also from Kelcee Everette from David Liu’s lab who is also developing a CRISPR-like approach called prime editing to correct the wrong letter or inset a missing letter in CDKL5 mutated genes. In both cases, much of the work focuses on improving efficiency (more copies of the gene fixed), specificity (not mess up with other genes) and figuring out how to make the CRISPR tools smaller so that they can fit the viruses used in gene therapies. These projects are like building new houses with completely new materials that had never been used before, and that so far are too large to fit well into the house. So timelines are unknown, but progress is very tangible. And it is quite amazing to see that one of the first diseases that these new technologies are being applied to is precisely CDD.
Multiple gene therapies in development
6. TWO SUPRISES THAT HAVE OPENED TWO IMPORTANT NEW DOORS
Not everything is taking longer than we thought. Some things are happening faster than we thought. We saw two great examples of this at the Forum.
Learning from evolution
Dr Ibo Galindo from the CIPF presented a fascinating evolutionary analysis of CDKL5 from the first single cell that existed and throughout the entire tree of life. It turns out that plants had CDKL genes but lost them (they have CDD!), and the original CDKL gene was CDKL5 but then it got duplicated twice leading to 5 forms. And it turns out that while all CDKL1-5 forms appear to be important to control cell skeleton, CDKL5 seems to have acquired new functions in vertebrates. There were two important conclusions: (1) there is probably functional redundancy among CDKL proteins, and (2) CDKL5 has a unique tail in vertebrates that suggest a new function not related to cytoskeleton.
And those conclusions provide a beautiful context to two projects coming from the lab of Dr Sila Ultanir at the Crick Institute that I summarize below. Sila’s lab was awarded the Lab of the Year Award by the Loulou Foundation in this last Forum, but I like to call them the “Lab of the Decade” because of their major ongoing contribution to the CDD field.
(1) Functional redundancy. Found the right paralog. Found a new therapeutic avenue.
A paralog is a gene that was born from a duplication of another gene, so now the two copies are so similar that they might have overlapping functions. This is what happened in SMA, the neuromuscular disease that is lethal in babies born without any functional SMN1 gene, that is now treatable because scientists found a way to use SMN2 (a paralog!) to compensate for the loss of SMN1.
Sila’s lab found that the most famous cytoskeleton target that we know for CDKL5, called EB2, wasn’t only controlled by CDKL5 in neurons, there seemed to be another kinase that could do the same job. And it turned out to be CDKL2! So now she is looking into the possibility of upregulating CDKL2 to compensate for the loss of CDKL5. And there is a potential to achieve this using an ASO approach. At the Forum we had a wonderful lecture by Dr Stanley Crooke, who founded and led Ionis (the company that made the first ASO treatment for SMA) and later created a non-profit organization called N-Lorem to make ASOs specifically for people with nano-rare diseases. And he explained that “CDKL2 has nice architectural features to make it suitable for overexpression” using ASOs. And he knows about this!
It has been quite unexpected to find out that we might get to follow the steps of SMA by finding a second gene that could do much of the work of the first gene (at least in neurons, because looks like in other organs it could be another CDKL). And this happened super-fast.
(2) Predicted to have targets beyond cytoskeleton. Found the right target. Found a new therapeutic avenue.
Dr Marisol Sampedro, working in Sila’s lab, had made the discovery last year that CDD could be partly a channelopathy because she found a new target for CDKL5 that is an ion channel. The channel is called Cav2.3, and when it is not phosphorylated by CDKL5 (as would happen in the deficiency) the channel stays too open, and the neurons stay too active. So what we would need is inhibitors. You can read about this in last year’s Forum recap (point 4, where I called this discovery a breakthrough).
One year later we had a new company standing on stage, called Lario Therapeutics, telling us that they are developing Cav2.3 inhibitors for CDD and for a disease caused by gain-of-function mutations in that gene (so they also have too much activity). Lario’s chief scientist explained that they are close to finishing the chemistry optimization and to start running safety experiments to advance their new drug towards clinical trials. This is a precision medicine approach to treat CDD and this seriously happened super-fast.
SUMMARY: MOMENTUM
We learnt this year that the first gene therapy is taking a bit longer that we thought, but many other things are happening faster than we thought, including having new therapeutic avenues (CDKL2 and Cav2.3) that were unimaginable for us barely 2 years ago. We also had several multi-year-long efforts all give us tangible results finally this year, like the two observational studies that have recruited as many patients as Phase 3 trials, or finally having beautiful in vitro and in vivo epilepsy CDD models of the type that pharma companies need. It feels to me that the pace of progress is accelerating, almost like that recruitment timeline for the CANDID study, and we have reached a powerful momentum.
That is exactly the same word that Stan Crooke chose when he stood on stage, and told us how “you can feel the momentum behind the science” in the CDD space. And I think that is a good word to capture what we saw this year.
And this momentum builds on two pillars that were also mentioned often during the 2023 Forum: determination and the spirit of collaboration.
Voice of the patient at the 2023 CDKL5 Forum
So I will leave you with quotes from three of the parents that spoke at the meeting that capture some these messages:
Cristina, from CDKL5 Spain, reminding us that “People with CDD are amazing and they deserve the best”.
Majid, from the Loulou Foundation, acknowledging the road to come with “We have moved faster and further than I expected thanks to all of you. And at the same time, that’s never as fast or as far as hoped for by the families”. He then called upon the audience to recommit with even more passion, more drive and more determination.
And Heike, representing the CDKL5 Alliance, who told us about his daughter and her personality, the efforts that all countries in the alliance and doing at the local and international level, and how “together we are stronger”.
I know I say this every year, but this was the best CDKL5 Forum so far.
I hope you enjoyed this summary. Please let me know your thoughts in the comments.
Ana Mingorance, PhD
Disclaimer: This is my own summary and key learnings, and not an official text about the Forum by the Loulou Foundation. I write these texts with the parents of people with CDD in mind, so excuse also my lack of technical accuracy in parts.
Dónde está la mutación de tu hijo en CDKL5, y qué nos dicen los gemelos
Una conversación que suele salir mucho en reuniones de familias CDKL5 es la posición de la mutación de sus hijos en el gen, por si eso nos ayuda a saber la severidad de la enfermedad cuando crezcan. Os resumo abajo un listado de publicaciones y sus conclusiones.
Una conversación que suele salir mucho en reuniones de familias CDKL5 es la posición de la mutación de sus hijos en el gen, por si eso nos ayuda a saber la severidad de la enfermedad cuando crezcan.
Yo sé que hubo estudios que decían que las mutaciones hacia el principio del gen son peores, y que si son mutaciones hacia el final “como tienes casi toda la proteína” dan lugar a cuadros clínicos más leves.
Pero también sé que hoy en día ya no se cree en esa correlación. Pero para estar seguros he mirado la literatura para ver si aún creemos que hay correlación entre mutación y fenotipo, o si no. Os resumo abajo un listado de publicaciones y sus conclusiones.
Nota para el lector no experto:
Genotipo es el tipo de mutación
Fenotipo es el cuadro clínico, los síntomas)
2004 - Mutations of CDKL5 Cause a Severe Neurodevelopmental Disorder with Infantile Spasms and Mental Retardation
Artículo original que identifica CDKL5 como la causa del síndrome. Describen una familia que tuvo 5 hijos, y eso incluye dos sin problemas, un varón que se les murió con 16 años con discapacidad intelectual several y epilepsia, y gemelas idénticas ambas afectadas. Eso es lo que llevó a los médicos a buscar la causa genética más que probable del problema de los tres hermanos, y encontraron que era CDKL5. Ahora eso eso, una de las gemelas idénticas tenía un cuadro clínico de “síndrome de Rett” (que resultó ser CDD) mientras que la otra, gemela idéntica, solo tenía trastorno de espectro autista. Basado en las descripción, con la misma mutación, el hermano tenía un fenotipo muy severo, una de las hermanas un CDD promedio, y la otra muy altamente funcional.
La familia 2 tuvo también dos casos, ambas hijos d ella misma mujer aunque de diferentes padres. Una considerada Rett atípico porque siempre estuvo muy afectada, y otra Rett clásico porque empezó mejor y a partir de los 3 años perdió uso de manos y dejó de adquirir más capacidades motoras. Misma mutación.
Con estas dos familias, sobretodo con las gemelas idénticas, está claro que sabiendo la mutación NO puedes saber si será más o menos afectado.
2012 - Recurrent mutations in the CDKL5 gene: genotype-phenotype relationships
Miran a niños que llevan la misma mutación en CDKL5 y si se desarrollan de forma igual. Ven que hay una mutación repetida (una missense, de cambio de letra) que produce casos más ligeros, pero todas las otras repetidas más severos. Y de esas las hay hacia el principio y hacia el final.
2012 - What We Know and Would Like to Know about CDKL5 and Its Involvement in Epileptic Encephalopathy
Explican “hasta ahora no se ha visto correlación clara de genotipo-fenotipo en casos de mutaciones en CDKL5. Algunas publicaciones sugieren que mutaciones hacia el final del gen dan lugar a cuadros clínicos menos severos, pero otros argumentan que la naturaleza de la mutación no tiene impacto en el espectro clínico. Por ejemplo el estudio de Weaving y colabores que describe dos gemelas idénticas con un fenotipo muy opuesto, una con un fenotipo que concuerda con Rett y la otra con espectro autista pero de discapacidad intelectual leve. Como se les miró la inactivdación del cromosoma X y ambas lo tenían normal, se piensa que su diferencia de fenotipo se debe a genes modificadores o factores ambientales"
2014 - Mutations in the C-terminus of CDKL5: proceed with caution
Algunos casos de mutaciones cerca del final del gen se ve que estaban mal interpretados, que se los había considerado patogénicos pero no eran porque eran heredados de los padres (todos llevamos muchas letras cambiadas en nuestro ADN, la inmensa mayoría de las veces no son problemáticas). Avisan de que las mutaciones en los exones 19’21 podrían ser falsa interpretación y tener cuidado con esos resultados genéticos.
Me pregunto si este descubrimiento también se juntó con la creencia de que mutaciones al final son menos malas. Estas no eran mutaciones, eran variantes que tenían esas familias igual que tenemos todos variantes de muchos genes.
2015 - Two Siblings With a CDKL5 Mutation: Genotype and Phenotype Evaluation
Dos hermanas, con la misma mutación (a principio del gen). La primera tiene retraso de desarrollo severo, hipotonía muy alta desde que nació, con problemas para comer con lo que lleva gastrostomia, y crisis refractarias. La segunda no tiene síntomas hasta 3 meses, cuando le viene la epilepsia y los síntomas. Hasta entonces ni hipotonía ni nada. Ya tras empezar las crisis si que deteriora, y consideran que tiene encefalopatía epiléptica. Al final la pequeña está más afectada que la mayor. Piensan que quizás sea por lo de la inactivación del cromosoma X, que quizás la pequeña tenga más neuronas con el gen mutado. Lo que importa es que con la misma mutación desarrollan distinto, y que la que empezó menos severa al final tenía la peor enfermedad. Con lo que no se puede saber.
2020 - CDKL5 Deficiency Disorder: Relationship between genotype, epilepsy, cortical visual impairment and development.
Este es un artículo de de los mayores especialistas de CDD en estados unidos. Explican que “los análisis de correlación de genotipo-fenotipo han sido difíciles en CDD porque no había suficientes pacientes y porque teníamos pocas mutaciones repetidas” y por eso juntan 92 pacientes, para ver si algo correlaciona, y concluyen que “ningún parámetro de la enfermedad correlacionaba con el genotipo”
2021 - Exploring genotype-phenotype relationships in the CDKL5 deficiency disorder using an international dataset
Usan una base de datos de 285 casos para ver si hay correlación entre el tipo de mutación y el desarrollo. La severidad de la enfermedad la encuentran igual en todos los tipos, si acaso si la mutación es trúncate hacia el final del gen (después del aminoácido 781) podrían estar un poco mejor cuando se les mira como grupo, o sea sabiendo que siempre hay diferencias individuales.
Describen algunas variantes individuales que están peor, pero a mi personalmente no me gusta interpretar así estos resultados porque una persona es combinación de muchos factores no solo la mutación en concreto con lo que el hecho de que el niño más afectado tenga la mutación X, no quiere decir que un segundo niño la vaya a tener igual. Eso es lo que vemos con hermanos, que salen distintos aún llevando ambos la misma mutación.
2022 - Factors influencing the attainment of major motor milestones in CDKL5 deficiency disorder
Usan una base de datos de 350 casos (la misma que en 2020 pero con más casos) para ver qué factores predicen el mejor desarrollo de las personas con CDD, sobretodo los que consiguen sentarse solos. Si que dice que si la mutación es trúncate hacia el final del gen (después del aminoácido 781), o ser niña en vez de niño, tienen mayor probabilidad de llegar a sentarse solos. A los que la primera crisis les empieza más tarde también tienen mayor posibilidad de llegar a sentarse solos. Pero cuando miramos los datos para ver cuanto más de probabilidad tienen de llegar a sentarse: un bebé que tiene una mutación sin sentido (de parada prematura) al final del gen tiene una probabilidad de un 70% de que se pueda sentar solo cuando tenga 5 años, y uno con una mutación sin sentido (de parada prematura) al principio del gen tiene una probabilidad de un 55% de que se pueda sentar solo cuando tenga 5 años. O sea no quiere decir que mutación temprana implica caso severo, o que mutación tardía (en el gen) anticipe que el niño o niña será de los que caminan y hablan.
ULTIMO:
Por último, el blog de genética de epilepsias resume que:
“Análisis de genotipo-fenotipo se han hecho pero no han conseguido ver ninguna correlación consistente. Y la observación de que gemelas idénticas tienen diferentes fenotipos enfatiza que juegan mucho papel otros factores ya sean genéticos, epigenéticos o ambientales"
Y esta es la conclusión con la que yo estoy más de acuerdo. Que no tenemos datos para pensar qué saber la mutación del niño anticipa su desarrollo. Al contrario, tenemos datos para saber que con la misma mutación pueden llegar a tener desarrollo muy variable.
Ana Mingorance, PhD
IEC2023 Dublin
The International Epilepsy Congress (IEC) is one of the largest epilepsy meetings attracting clinicians, researchers and the pharmaceutical industry. In 2023 it took place in Dublin, in early September. I review the progresses towards treating the cause of the rare epilepsies / DEEs
The International League Against Epilepsy (ILAE) hosts every two years an international epilepsy congress (IEC) that just took place in Dublin. The main conference in the field is still the American Epilepsy Society meeting that takes place every year in early December (check out my summary of the 2022 AES meeting) but the IEC is a close second.
This text is not meant to be an exhaustive review of all what was discussed at the IEC. These are my highlights, as a rare epilepsy scientist who has been attending this type of conference for many years. Fun fact: did you know that in 2013 this conference was also held in Dublin? That was the year I decided to leave pharma to be able to work directly with rare disease patient groups. So this conference was very special for me, being back at the IEC in Dublin exactly 10 years later.
Here are the 9 messages that grabbed my eye during the IEC2023 conference, plus a bonus message. As usual, I focus on the development of treatments for rare epilepsy syndromes or DEEs.
1. Voice of the patient in the agenda
Amber Freed from SLC6A1 Connect
The IEC organizers did a great job at having many of their sessions open with the voice of the patient. And not just in the small rooms.
Amber Freed from SLC6A1 got to open the Presidential Symposium of the conference. The day before we heard from Gabi Conecker about SCN8A, and both supermums told the audience of clinicians about how the complexity of the DEEs mean that (1) we need coordinated multidisciplinary care, and (2) doctors need to listen to families. Listen not only because it is a nice human thing to do, but also because people with DEEs can do strange things that is not clear what they are (seizures? stereotopies? movement disorder?) so doctors really need to listen to families to understand what’s happening.
These are not new messages, but it is a new stage: the BIG stage. And such change is important to normalize the message that families are central to how we take care of people with DEEs, and that for that to happen we need to regard them as peers, including sharing stage. No more sitting at the back of the room listening to clinicians.
2. Comorbidity doesn’t mean comorbidity
Dr Scott Demarest from Colorado explained this very well. He said that:
We always talk about “comorbidities” because we are epileptologists, so everything that is not epilepsy is a comorbidity. But really these are also defining features of a syndrome, not necessarily set off to the side.
Dr Antonio Gil-Nagel from Madrid also explained later in the conference that only 3% of patients with DEEs have only seizures, so almost everyone has many more things going on: intellectual disability, developmental delay, endocrine problems, motor problems, language problems (the % of non-verbal people with DEEs is high!). He said that in his practice, families often report behavioral problems as their top priority, with aggressivity and impulsivity causing much stress to families.
I’ve also heard this a lot from families (Syngapians and PHD19 girls, I’m thinking of you here)
For me it was clear at the IEC2023 that this “comorbidity doesn’t mean comorbidity” message is sinking in, not only because we heard it from everyone (parents and doctors on stage) but also because there were several sessions dedicated to some of the non-seizure aspects of DEEs. And remember that this is an epilepsy conference! not a neurodevelopmental meeting, so this is really a big step towards helping look at these diseases more globally, which is an absolute requirement towards being able to manage them more globally.
3. Changing the therapeutic goal and how we talk about DEEs
Dr Elaine Wirrell from the Mayo Clinic explained the therapeutic goal for DEEs and how it is different from managing a patient who only has epilepsy. For epilepsy it usually is to try to get to seizure freedom with no (or manageable) side effects. For DEEs, which are so tough to treat, the goal of zero seizures would mean excessive sedation. Instead, the therapeutic goal should be:
“target the most problematic seizures, avoid therapies that might worsen seizures, avoid excessive polypharmacology, and manage the comorbidities to maximize the quality of life”
But that is now, when all we have is anti-seizure drugs and some anti-anxiety or anti-depressant medications, melatonin and others to sleep… all symptomatic! (and rarely very effective). The therapeutic goal will change once we have access to real disease-modifying therapies, which I talk about in some of the next sections.
Dr Elaine Wirrell also gave another very good talk where she reminded collages that words do matter, and to please stop using the term “catastrophic epilepsy”, in particular when explaining to parents what their child has been diagnosed with.
I have a friend (and Dravet dad) called Julian who would often start his presentations with exactly that word, “catastrophic”, as the single word in a slide. And would pause there. To let it sink into the bones of everyone in the audience. Catastrophic means that nothing can be done, it erases all hope.
And Dr Wirrell was fantastic at reminding us that catastrophic is not a medical term, and that:
“words matter! We CAN make a difference for people with developmental and epileptic encephalopathies and they ARE NOT catastrophic!”
She was a fantastic speaker.
4. What is disease modification?
Another of my favorite speakers was Dr Scott Demarest from Colorado. He spoke about how disease-modification “means we have to care about things other than epilepsy” and commented on some of the efforts in development to measure those other symptoms during clinical trials. As he explained, a clinical trial with a disease-modifying therapy (like those correcting a gene problem) has to be different from epilepsy clinical trials. This is not about counting seizures for 12 weeks, this is about counting a lot of new things for a lot longer than 12 weeks. For some of the syndromes we might not even be able to use seizure frequency as the primary endpoint (not enough or hard to count), but that’s ok, we are now seeing more assessments developed for many other disease aspects.
Dr Scott Demarest on disease-modifying trials for DEEs
Dr Demarest explained that disease-modifying therapies are treatments that target the fundamental basis of a condition, and that aim to improve the entirety of the phenotype, but do not necessarily mean “cure” in the sense of erasing the disease completely. But most DEE families that I know already call “cure” a treatment that targets the gene and improves several of the symptoms, so I think we agree on the goal: tackle the biology, and give the body a chance to do its best.
And I would like to take a second to pause here, and let you all realize how this conversation is an inflexion point in the field. This is the International League Against Epilepsy talking about disease-modification (!!), talking about cures, about fixing genes, about getting those treatments through clinical trials. All center stage.
As Prof Ingrid Scheffer from Melbourne said later in the conference, “if you came from the Stoke session, you know that gene therapies are here and now”.
Let that sink in.
It is not just the Stoke clinical trial. It is the whole field. It is a whole pipeline of treatments designed to target the genetic problem of several of the DEEs (many not presented at the IEC2023). This is an inflexion point in the field. Disease-modifying therapies are here and now.
5. Talking a lot about outcome measures
The imminent arrival of therapies that can do more than reducing seizures is changing how we think of clinical trials. Now we want to measure much more, and measuring more means having the scales or assessments to measure those things. That’s called clinical outcome measures.
Outcome measures are not a synonym with a comorbidity, they are scales to measure that comorbidity. It is sometimes confusing, also if we add the word endpoint which is the “goal” of moving that scale. Here is a simple example:
Imagine that you want to gain muscle mass to be able to get better at weightlifting:
The two “comorbidities/symptoms” to measure: weight and strength
Outcome measure 1: scale that can discriminate muscle and fat
Endpoint 1: two more Kg of muscle in X months
Outcome measure 2: measuring the maximum amount of weight you can lift for one repetition
Endpoint 2: increase the weight that you can lift for one repetition by X Kg
So as you can see we start getting into the technical aspects of clinical trials, like the specific assessments that we should use, which by the way should meet the requirements for being used for drug assessment, and also deciding what the goal change should be (the endpoint).
By the way, Clinical Outcomes Research is like a whole field of science! And here we have the clinicians who treat patients with DEEs rolling up their sleeves and getting fully fluent on the technicalities to develop outcome measures and endpoints that can help us run the new clinical trials. IEC2023 looked nothing like the 2013 edition, which was all about using approved anti-seizure drugs better. I tell you, this is an inflexion point in the field. And there is much more community involvement in the field, counting both clinicians and patients, than ever before.
This also opened up interesting debates: not all assessments are equal, so conclusions should NOT be also considered equally. Some of the companies with drugs approved to treat seizures in DEEs are using surveys or scales that capture the parent’s perception of how their child is doing to say that their drug can improve behavior or cognition. A doctor from the audience said very strongly (and rightfully) that you cannot claim that a drug can improve cognition if you don’t use psychological assessments to confirm that. So by all means you should listen to parents to understand the patient experience on a particular drug, but you need to go beyond that and involve neuropsychologists if you want to make certain claims of efficacy.
In the example above about weightlifting, it is not about whether you feel stronger, or your coach thinks that you are stronger, it is about whether we can verify that increase in muscle mass and performance.
Prominent stands with drugs for Dravet syndrome and LGS
6. A big part of the agenda is about DEEs…but mostly about three of them
DEEs were part of pretty much every session at the IEC2023, and often the only subject of entire sessions.
Much of this is because we are diagnosing many more of the syndromes, and learning about them, so at some point the discussion on seizure types (focal onset and generalized) takes as much space as the discussion about different syndromes.
But part of this is because pharma companies sponsor many of the sessions, and they of course want to talk about the diseases that they have drugs for. So with Epidyolex and Fintepla and Diacomit we got to hear a lot about Dravet syndrome, Lennox-Gastaut syndrome, and TSC. You could hear about these diseases in the exhibit area and also in all the sessions that companies sponsored.
And this is great as a field! But it was painfully narrow to focus so much on those few diseases. I understand that this is because a big focus of the conference is to learn about the medicines that are available and how to use them, and we now have several medicines for those syndromes. And also these are some of the most common syndromes so clinicians end up seeing many more of these patients than those with more rare DEEs. But I still believe the agenda skewed a bit too far.
Prof Ingrid Scheffer from Melbourne gave us a number that was truly eye opening: there are 925 monogenic epilepsy genes, and 825 of those explain about half of all DEE cases (others might not be genetic or a gene has not been found yet). That’s a lot!
Compare that to the focus on three syndromes.
I know that there were several sessions on genetics and I missed those (I read Dr Dennis Lal on Twitter to know what was said: @LalDennis). But those sessions focus largely on genetics as a way to DIAGNOSE, not as a way to TREAT. I think the only exception was the talk “Single gene approaches to precision medicine” precisely by Dennis Lal.
What would I like to see at a conference? I would like to see more discussions on DEE as a class, including all of the very rare genetic ones that we keep discovering year after year. Rare genetic DEEs have two things that make them different from other DEEs, so they need more space in this type of conference or we will not be prepared to treat them:
1) They are genetic, so we can think of ways to develop disease-modifying treatments for every single one. Isn’t this great? We might not know the best drug to treat a teenager with LGS but it is very straightforward to know what a child with a genetic DEE needs – the tricky part is to make that treatment. Some will be small molecule drugs to correct an ion channel activity (if that’s the cause of their DEE, like for SCN8A) or an ASO to change the protein expression levels (like for SYNGAP) or a gene therapy to put a gene back (like for CDKL5). Yes we will still help these patients with anti-seizure drugs, but we should talk more about all of the DEEs that have known genes and the different ways we could try to correct that problem and how we can turn those ideas into realities. If we do that, we will bring much more biotech (not just old pharma) to the table, and we really need that.
2) All genes produce a spectrum of phenotypes, and this impacts clinical trials. The clinical diagnoses for DEEs describe an electroclinical profile that is relatively homogeneous, that’s why clinicians have given it a name. But the genetic diagnoses of DEEs all produce a spectrum of symptoms and prognosis. That’s why you can have both Lennox-Gastaut syndrome as your epilepsy type, and CDKL5 deficiency disorder as your genetic diagnosis for example. So clinical trials for the majority of the DEEs, which are genetic diagnoses, are going to be a lot harder, and need more complex trials, relying more on non-seizure outcome measures. They cannot be left off the stage as we focus on Dravet syndrome and LGS and a few other clinical diagnoses (the ones that are good for epilepsy trials).
All that said, I think this is also about a field having been studied for longer. I liked how Dr Rima Nabbout from Paris acknowledged that we were talking a lot about Dravet syndrome at the conference, but also WHY it has become likely the most popular epilepsy syndrome at the meeting. She said:
“Dravet syndrome is emblematic because many years ago there were families that decided that they wanted to work hand in hand with the clinicians, with the pharma industry, and with the whole world”.
And she is 100% right. 12 years ago I started working with Dravet syndrome families to figure out how to make the disease better known to industry. There was only Diacomit and only in Europe. There were no plans for more trials, no companies interested. Dravet syndrome today, is many of the monogenetic DEEs in 5-10 years.
7. Adult neurologists step up
As we get better about diagnosing DEEs, and about taking care of these patients, we have more and more adults with DEEs. This contrasts with adult neurologists receiving a lot less training on these “childhood epilepsies”. And ironically, we are now running trials in patients under 18 years of age in these populations so some drugs get approved only for 18 and younger, or approved for more ages but without data in adults.
And to quote Dr Nabbout again:
“if children are not small adults, it is important to know that adults are not big children”
As patients grow, often the main concerns about their disease change. The parents of a small child with a DEE might worry mostly about seizures, but the parents of a 25 year old with a DEE are more concerned with aggressive behaviors and their son’s declining ability to walk independently, for example. It is important to also make sure that the usual multidisciplinary (and more coordinated) care of pediatric patients is also applied to adults after they transition to adult care, but this doesn’t happen so often. The International League has created a taskforce on transition (from pediatric to adult medical care), and it is led by Dr Nabbout. This is great.
8. Hot drug #1: XEN1101
This drug is not in development for any DEE, but looks great for focal-onset and generalized seizures (currently in Phase 3 trials for both) and is a good example for the type of small molecule drug that we could develop for different types of DEE. XEN1101 opens up an ion channel called Kv7, so that more potassium can flow through. There is one DEE caused by mutations in that very same potassium channel gene, and patients need exactly a drug that can open up the ion channel Kv7 because they only have half of the number that they need (KCNQ2 haploinsufficiency).
This is one of the most exciting drugs in development for epilepsy right now, and could be very useful for patients with KCNQ2-related epilepsy once it gets approved. For many of the DEEs caused by mutations in ion channels, small molecule drugs like XEN1101 could be the way forward.
9. Hot drug #2: STK-001
The second main strategy for genetic DEEs is to use advanced therapeutics like ASOs and gene therapies to tackle the genetic problem. That is what the ASO STK-001 from Stoke Therapeutics is doing: upregulating the mRNA for SCN1A so that patients with Dravet syndrome can have more of the ion channel. Dravet syndrome is largely caused by SCN1A haploinsufficiency so this is the ideal type of treatment to try to treat the entire syndrome.
Stoke initiated the current Phase 1/2 trial about three years ago, first with only a single administration to find tolerable levels, and then with multiple administrations of increasing doses. This is the first trial of its kind for DEEs, so it has allowed us to learn many things, like how long to wait before expecting improvement, but also which types of improvements and how large. The IEC2023 was the first large conference where Stoke presented their clinical trial data, after announcing the latest results this summer. And it is safe to say that they have finally found the good doses and the treatment is looking quite good. To quote Dr Dennis Lal recap of the data presentation:
“impressively, not only many patients have >50% seizure reduction but also cognitive measures show improvement.”
And that is what makes this treatment so special, the possibility to improve symptoms beyond epilepsy, in a way that can be quantifiable in clinical trials, because it is not acting on a symptom but on the disease itself.
Or as Dr Scott Perry from Texas said:
“finally a treatment showing evidence of true disease modification for Dravet syndrome!”
And I want to remind you that Dravet syndrome today, is many of the monogenetic DEEs in 5-10 years.
There is more to treatment development for DEEs than what we saw
At the last AES meeting I wrote that:
“We have crossed the line of developing treatments for symptoms, to developing treatments for the cause of the disease. And the line of focusing on a few syndromes only (those with the most patients), to seeing treatments in development for many more. That’s escape velocity.”
The IEC2023 meeting capture the first part very well, but missed the mark on the second. It captured very well the change in mentality and priorities of the clinicians and their representing organizations, perhaps even better than at AES, but barely scratched the surface of the true therapy development pipeline for DEEs, for many DEEs, so I recommend you to read my summary of the last AES meeting in this website, and see you in Orlando for AES2023.
I hope you enjoyed this update, let me know your thoughts in the comments,
Ana Mingorance, PhD
Disclaimer: I write these texts with the parents of people with rare epilepsy syndromes in mind, so excuse also my lack of technical accuracy in parts.
AES 2022: genetic epilepsies reach escape velocity
The American Epilepsy Society (AES) meeting is the largest epilepsy meeting of the year, and because it takes place every month of December it also serves as an annual review on the understanding and treatment of epilepsies. I review the progresses towards treating the cause of the rare epilepsies / DEEs.
PART 1 – ESCAPE VELOCITY
I often write a summary of the main lessons from the American Epilepsy Society meeting, but this year there was so so much about the rare epilepsy syndromes that I was not able to follow the main conference, there was barely enough time to follow all what was happening around the syndromes (which you also see referred to as Developmental and Epileptic Encephalopathies, or DEEs).
So I will focus my summary this year on the development of therapies for the rare epilepsy syndromes.
The conference started really strong, with the SYNGAP1 Conference the day before the main AES conference, where we learnt from Dr Jacquie French that for the first time, in 2022 we had more trials in rare epilepsies than in adult epilepsy. Even if people with rare diseases represent only about 20% of the total number of people with epilepsy, now they have the lion’s share of the trials. This is huge news!
And during the main AES meeting it was clear that indeed, much of the attention is currently on the rare epilepsies, with much of the agenda dedicated to genetic diagnostic, understanding how the adults with rare epilepsies look (so we can find them and develop treatments!), and much much research on the development of treatments. That’s why I believe that this year, with the milestone of having as many trials in syndromes as in regular epilepsy, we have crossed a line and likely reached escape velocity. Because it is not only about the two or three “famous” syndromes anymore, or about anti-seizure drugs being tested for the syndromes. We have crossed a different line.
At the other side of this line are treatments that target the cause of the monogenetic epilepsies. And those treatments look very different than classical anti-seizure drugs, and those trials also look different. We have crossed the line of developing treatments for symptoms, to developing treatments for the cause of the disease. And the line of focusing on a few syndromes only (those with the most patients), to seeing treatments in development for many more. That’s escape velocity.
PART 2 – TREATMENTS TO CORRECT THE CAUSE OF 8 SYNDROMES
Here are some of my highlights for eight of these monogenetic syndromes as an update to families. There are more diseases, don’t be discouraged if yours is not covered in this summary, I couldn’t do all of them.
SCN1A
Dravet syndrome is the (mostly) monogenetic syndrome that has received the most attention so far, and as a result the one with the most approved therapeutics. We saw many updates at AES about the approved drugs, and presentations about ongoing clinical trials with soticlestat (Phase 3) and EPX-100 (Phase 2). But I will focus on the treatments for the cause of the disease.
One of the most anticipated news of this congress were the early results from Stoke using an antisense oligonucleotide (ASO) called STK-001 to increase the good copy of the gene SCN1A. That’s because Dravet syndrome is a haploinsufficiency, which means that one copy of the gene is bad but one is good and can be exploited to produce enough protein from that one copy. That’s what Stoke is doing. After completing lower doses as part of the safety protocol for this type of therapy, Stoke has recently started administering higher doses to patients with Dravet syndrome and seen seizure frequency reduction and also improvements in non-seizure aspects of the disease. There is more information about this on their website. This is still the first small group of patients receiving enough dose to measure efficacy, and the results look very positive to me.
Another company using a similar antisense (ASO) approach, but directed to the SCN1A gene (not the RNA like Stoke), is CAMP4, that presented at AES how they are getting their antisense treatment ready for clinical trials (this is the antisense that OPKO was developing).
At the SYNGAP1 pre-meeting we saw a fantastic presentation from Tevard Bio about their approach to attack Dravet syndrome using different approaches all directed to the SCN1A RNA. And while Tevard still needs more time to get to clinical trials, Encoded Therapeutics is very close to starting trials with their gene therapy using a virus to increase SCN1A expression from the good gene copy, and presented at AES their work on finding good clinical scales to measure non-seizure symptoms of Dravet syndrome (the AES links are broken, so you will have to take my word for it). Encoded is preparing to start trials in very young children with Dravet syndrome (not yet started), and their gene therapy has the potential to change the developmental trajectory of these kids. The challenge is to get the right scales to capture that efficacy, so both them and Stoke have observational studies ongoing to validate those scales.
And Dravet syndrome also has at least two companies developing activators for the Nav1.1 channel, Lundbeck and Xenon. These are drugs, what scientists call small molecules, and not genetic therapies. From these two, Xenon has the most advanced program (another broken AES link that I cannot use), and I could see the Dravet syndrome space developing similar to SMA and have first an ASO approved, then a gene therapy, and finally a small molecule, all restoring sufficient levels of the sodium channel currents.
Shout-out to Veronica Hood, Scientific Director of the Dravet Syndrome Foundation who gave a fantastic talk about the impact of the patient groups in Dravet syndrome with major roles as conveners, educators, funders of early research and resource curators.
SCN2A
SCN2A is another sodium channel very important for neuronal functioning, so important that mutations that cause too much activity produce one of these developmental and epileptic encephalopathies (I will call it SCN2A Gain-of-Function) and mutations that cause one copy to not function also produce a syndrome with epilepsy and cognitive and behavioral manifestations (I will call it SCN2A Loss-of-Function). That means scientists need to find ways to turn off the channel or the gene for the first group, and find ways to boost expression of the good copy for the second group.
The company Praxis is doing just that, and working at both approaches with different types of treatment modalities. The most advanced program is called PRAX-562 and is a drug (a small molecule) designed to inhibit only two channels: Nav1.2 (that is the one produced by SCN2A) and Nav1.6 (produced by SCN8A). This drug looks really promising for people who carry mutations in either one of these channels that causes the channel to work too much, so it is for the Gain-of-Function mutations. And Praxis is about to start clinical trials for these two syndromes after completing Phase 1 in healthy volunteers.
What’s interesting is that Praxis also has a second program for people with SCN2A Gain-of-Function, and that is an ASO called PRAX-222 developed in collaboration with Ionis, and it is also getting ready to start clinical trials. So 2023 is going to be a very good year for SCN2A Gain-of-Function. And Praxis is also working on an ASO to do the opposite: increase SCN2A expression. This is a treatment for the SCN2A Loss-of-Function patents and it is still at earlier stages of development.
I will mention Praxis more times in this summary because they are working on more syndromes, and I want to highlight that this company has parents of kids with rare DEEs working with them as part of the team, and this is a very good sign. When we have scientists working within patient organizations, and patient advocates working within biotech/pharma companies, we can start breaking down many of the communication and cultural barriers between both worlds and developing better medicines.
SCN8A
SCN8A is the gene that encodes for another very important sodium channel, called Nav1.6. This is a channel that makes excitatory neurons fire, so the most common mutations are the Gain-of-Function type, that make those neurons way too active. What we need is clear: reduce the channel expression, or inhibit the channel activity.
This patient community has done a really good work at trial readiness, and we saw at least three therapeutic programs at AES (I might be missing some):
The clinical trial from Praxis with the drug that blocks Nav1.2 and Nav1.6 that is about to start.
A clinical trial with another drug, developed by Neurocrine and Xenon, that is only inhibiting Nav1.6. This one is already in clinical trials!
An ASO that we saw in posters and that reduces SCN8A expression so that there is not too much channel. This one is still in animal studies.
I look forward to the conference on SCN2A and SCN8A next spring because it looks like we will have many news about clinical trials for these syndromes!
KCNQ2
And from sodium channels we move now to potassium channels. This gene is very famous in epilepsy, because if you have a very young baby, under one month of age, that starts having seizures, the most likely cause is mutations in this gene. What these kids need is openers for this potassium channel, activators.
And there are two companies developing activators, one is Xenon, who are taking an older drug (ezogabine) that was taken off the market back into the market this time for KCNQ2 epilepsy. The drug is currently in Phase 3 studies, which means that if the trial is successful it will lead to an approval for KCNQ2. The second one is also an activator, from Knopp/Biohaven, that is currently in Phase 1 studies and will then move on to trials in patients with KCNQ2 epilepsy.
As with SCN8A, we have gone from not having trials to having even competing trials with drugs designed to correct the channel problem, either too much (Nav1.6) or too little (the potassium channel Kv7) activity. And I mainly look forward to seeing the potential benefit of these drugs past the very early years, once children with KCNQ2 mutations often stop having epilepsy but still have the developmental impacts that are due to the channel malfunction.
SYNGAP1
There is so so much going on for SYNGAP1 that I cannot summarize it all here. The pre-AES SYNGAP1 conference was fantastic, with many families in the room (and also all of the famous clinicians and many companies). There are iPSC models and mice and rats, I really liked the rat model and how it can be used to study drug response for seizures, sleep and cognition. They also have an idea of the SYNGAP1 isoform that is needed for gene therapies, and the “reversibility experiment” of putting SYNGAP1 back into adult mice has been done and it looks very positive. There is also a lot of work ongoing around patient data, like Ciitizen to see natural history, and an outcome measure for communication called ORCA being adapted to SYNGAP1 with funding from FDA. It’s all looking ready for therapies to be run through trials! There are two very strong SYNGAP1 foundations working to get trial ready and it shows.
And talking about therapies, there are several ASOs in development for SYNGAP1 and other modalities of gene therapies. What happens in this disease is the classical haploinsufficiency: one good copy of the gene, and one bad copy of the gene. That’s why the focus of these therapies is in promoting more expression from the good copy so that synapses can go back to working well again.
Praxis is working on an ASO to increase SYNGAP1. It is called PRAX-090 and in recent presentations Praxis says they hope to nominate a candidate in 2023. A “candidate” is when the company considers that their experimental drug or antisense is “good enough”, and at that point they stop tweaking it and start generating the animal safety data that is needed for trials, so it usually takes 1 to 1,5 years from the time of candidate nomination to starting trials.
Stoke Therapeutics, the company developing the ASO for Dravet syndrome (SCN1A) also has a program for SYNGAP1 using the very same approach, and they have partnered it with Acadia. And another company called CAMP4, that is also working on upregulating SCN1A for Dravet syndrome, presented at AES their early work towards also developing an ASO approach for SYNGAP1. And there are some additional academic and industry (Ionis) efforts to develop ASO treatments, so it is likely that SYNGAP1 families will get multiple options of clinical trials to increase protein levels, which should be a treatment for all the different symptoms of the disorder.
But antisense oligonucleotides are not the only approach to rescue SYNGAP1 expression. At the pre-AES conference we saw how Tevard is applying some of their gene therapies to increase expression from the SYNGAP1 RNA, and how scientists at Penn are using a CRISPR-like approach (called dCas9) to increase expression from the good gene copy. These are all very early stage efforts that are likely to reach clinical trials after the ASO treatments.
So you see, a lot going on, and not everything is presented at AES! And shout-out to Marta Dahiya, a clinician and SYNGAP1 mum who gave a very interesting talk during the main AES conference about digital natural history studies using platforms like the one from Invitae/Ciitizen.
STXBP1
Like the one before, STXBP1 is also a synaptic protein that when mutated causes haploinsufficiency: one good copy of the gene, and one bad copy of the gene. And the main therapeutic strategy to target the cause of the disease is therefore to promote more expression, often by exploiting the good copy, so that synapses can go back to working well again.
At AES there were several presentations around documenting the natural history of the disease, and also on validating scales to be able to run trials that count more than just seizures. The patient organization is working on a very large natural history study designed to validate outcome measures, and has recently announced natural history efforts by the companies Capsida and Encoded Therapeutics. These are gene therapy companies, the ones that use a virus to deliver the therapy to the brain, so we can imagine that gene therapies for STXBP1 are being developed even if they haven’t yet been announced.
There is also a known collaboration between the company Ionis and the Prosser lab to develop and ASO to restore STXBP1 expression, and at AES we also saw a presentation from the company Q-State about an antisense program to restore STXBP1 expression, which is still at early stages.
So it looks to me like STXBP1 disorder, like SYNGAP1 and others, have multiple companies working unannounced on therapies to correct expression of their faulty gene and that by next year AES there will likely be many more projects that have been officially announced. I very much look forward to that!
PCDH19
This is an interesting gene, because it encodes for a protein that makes cells touch each other to connect. It is in the X chromosome, so in females half of the neurons express the good X chromosome and the other half the one with the mutated PCDH19. That means that they have two type of neurons, with only half expressing the cell touch protein, and that is a problem because for neurons to touch each other they need to have the same code: either they both have PCDH19 or they don’t. What doesn’t work is only some having it. That is why in males, that express their only X chromosome in all neurons, mutations in PCDH19 don’t cause disease, because all neurons express the same code: no PCDH19.
There are at least two ASOs in development to make all neurons have the same cell touch code by eliminating expression of PCDH19 in all neurons in girls/women with PCDH19-Clustering Epilepsy. The first one is from Praxis, and is called PRAX-080 and as for SYNGAP1 the company has recently communicated that they hope to nominate a candidate in 2023 (the fully optimized ASO that then gets prepared to go to clinical trials). And the company Ionis is collaborating with the Parent lab to also develop an ASO that will achieve the same result: make female neurons like make neurons by knocking down PCDH19.
I like that for PCDH19 we have had clinical trials with small molecules (ganaxolone Phase 2) so we know for sure that seizures are countable and how a phase 2 trial could look. That helps de-risk the field for future trials, when companies will likely still count seizures as the main symptom for drug approval in addition to including scales to measure improvements in behavior and other domains – just as currently for Dravet syndrome.
CDKL5
CDKL5 Deficiency Disorder (CDD) is another DEE where the gene is in the X chromosome, but in this case the protein is a kinase and the problem is not that neurons don’t connect with different neurons, but that half of the neurons are missing the very important function of the protein CDKL5. The therapeutic goal is to put that protein back, with the added challenge that because only one CDKL5 copy is expressed in each neuron (only the good one or only the bad one) we cannot use many of the strategies used for haploinsufficiencies.
I dedicated a recent update to CDD from the CDKL5 Forum 2022, so please see that link to read how the first gene therapy trials to bring a new copy of CDKL5 to the brain of people with CDD might start as early as next year. And 2022 was a good year for CDD, we had the first drug approved for the disease: ganaxolone (approved by FDA, pending EU approval). So this year we had a new player in the big AES exhibit, with the large stand for Marinus highlighting their data for ganaxolone in CDD and helping educate the medical community about this rare epilepsy that now has a treatment.
As for PCDH19 epilepsy, CDD has very clear epilepsy that has been used already in clinical trials as the main measure for efficacy and that will help de-risk the upcoming clinical trials with therapies directed to correcting the cause, which should improve seizures as well as non-seizure symptoms (which are so much harder to measure). And like for other syndromes, what was talked at AES was just the top of the iceberg, and there are many more treatments in development for the cause of the disease that were not presented at the main medical conference. Check out the Forum update for that.
3 – IN CLOSING
If you are a rare disease parent you might have only read the section about your loved one’s disease gene. So I encourage you to do two things:
One, please remember that companies often don’t talk about their projects until they are close to trials, and not all present at AES, so what you read above is only a snippet of what’s going on in reality (iceberg!).
Two, whether you are a parent or a scientist, step back and look at all the syndromes and the global picture that emerges: I have only talked about the treatments in development to address the cause of the syndrome, not the symptom. Long gone are the times when there were no trials in the syndromes so we didn’t have good clinical evidence about anti-convulsant drug efficacy in them. And we are even closing chapter two, the one of cannabidiol and the other anti-convulsant drugs being tested and approved for syndromes, although their activity is not specific to the syndromes. What you are seeing here is different. You are seeing multitude of programs to block or open channels, to increase expression of good gene copies or reduce expression of bad ones, and several are already in clinical trials in patients. We are closing chapter two, and reading from chapter three… the one with treatments for the cause of the disease reaching clinical trials. And the speed at which this is happening, and how broadspread it is across different syndromes, is unstoppable. We have reached escape velocity.
Ana Mingorance, PhD
Disclaimer: I write these texts with the parents of people with rare epilepsy syndromes in mind, so excuse also my lack of technical accuracy in parts.