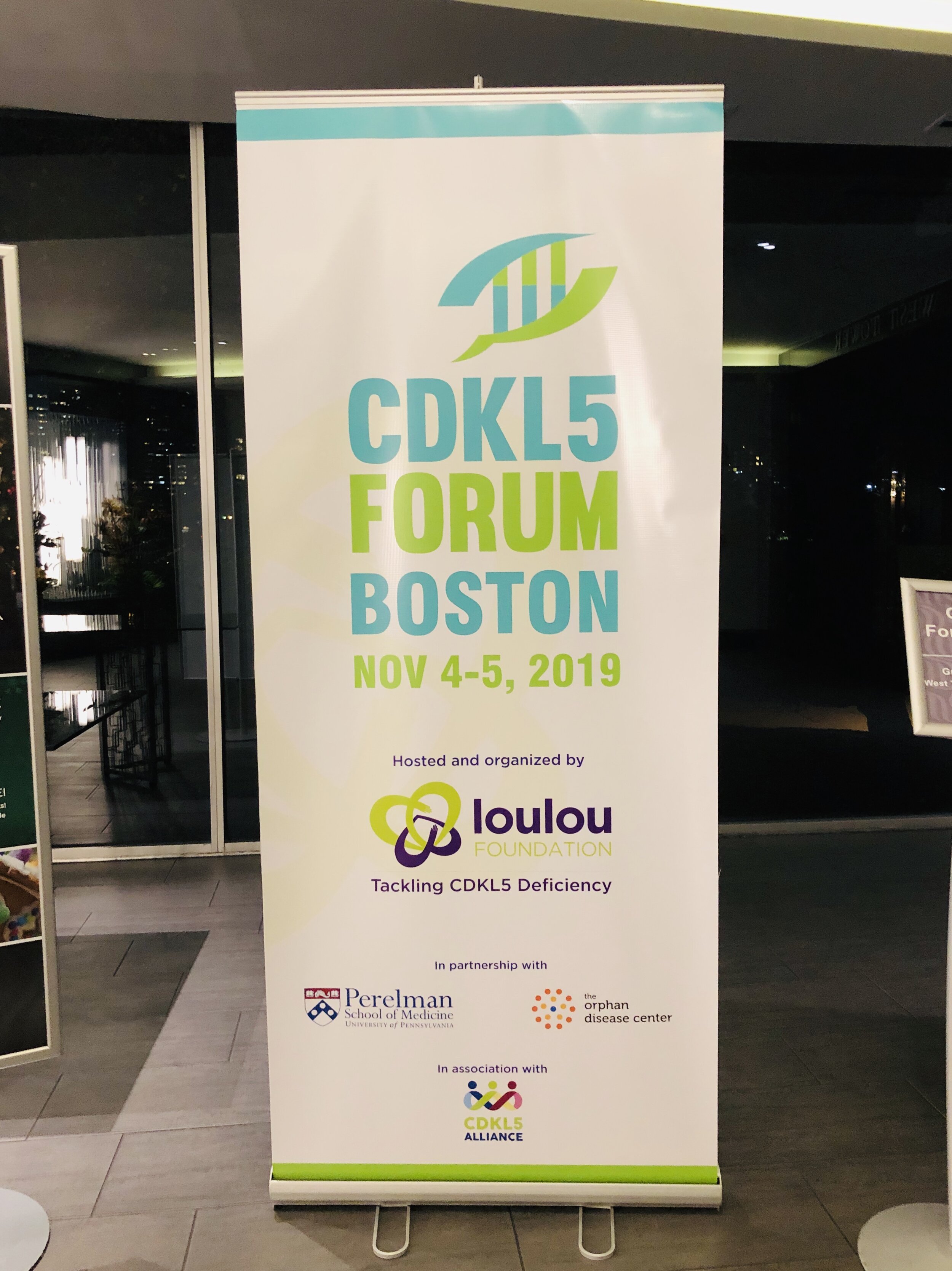Epilepsy Insights
Top 5 insights from the American Epilepsy Society meeting (2019)
The American Epilepsy Society (AES) meeting is the largest epilepsy meeting of the year, and because it takes place every month of December it also serves as an annual review on the understanding and treatment of epilepsies. AES 2019 was the year of genetic therapies for the developmental and epileptic encephalopathies. This article highlights what I found the most interesting at the AES 2019 meeting.
Versión en español en este enlace
The American Epilepsy Society (AES) meeting is the largest epilepsy meeting of the year, and because it takes place every month of December it also serves as an annual review on the understanding and treatment of epilepsies. I look forward every year to the first week of December for this reason.
I look for therapies for rare genetic epilepsies, and in the recent years this area has exploded to take over much of the AES meeting.
Two years ago, there was already growing attention for the orphan epilepsies* and everybody was excited about cannabidiol and fenfluramine. It was starting to become clear that there are just too many genetic syndromes to try to diagnose them one by one, so we need to run genetic epilepsy panels as soon as possible in children. AES 2017 was a great meeting, but I was still hoping there would be more of a connection between treatments and genetics. That we could move beyond genetic diagnosis and towards genetic treatments.
And we made a big jump in that direction at the American Epilepsy Society meeting in 2018. I wrote in that article:
There was one key progress visible at the AES 2018 meeting that defines a before and after moment in the field of epilepsy, and this is the arrival of non-pharmacological therapies for treating epilepsy.
That before and after was tremendously clear during this year’s conference. During AES 2019, the sessions on genetic therapies and antisense oligonucleotide (ASO) therapies for rare epilepsies were filled to full (standing) capacity and the organizers had to close the doors. The epilepsy field, which has been until recently dominated by pharmacology, has embraced the technologies of biotech and we have officially entered a new era.
Before I review the main take-home messages from the meeting, I want to clarify two terms that I will be using:
Developmental and epileptic encephalopathies. We don’t need to struggle with calling them “genetic epilepsies” “rare epilepsies” or “orphan epilepsies” anymore, always adding the message that “they involve much more than just epilepsy”. Now the field has a name. We are talking about the developmental and epileptic encephalopathies (DEEs), where not all are genetic (hello LGS!) but they all have a strong neurodevelopmental component that is not 100% due to the epilepsy. The term was introduced by the ILAE in 2017 and it is staying.
Genetic therapies. When we talk about gene therapies, we talk about using genes as a therapy (as in the classical AAV carrying a healthy copy of a human gene). But it is clear that our therapeutic armamentarium to target genetic diseases goes beyond that. Dr Barry Ticho from Stoke referred to their oligonucleotide therapy as a “genetic therapy”, because it treats the genetic problem even though the therapeutic agent does not deliver a gene. I loved the term, so I will use it as well to incorporate all the different DNA and RNA-targeting therapeutics regardless of them using virus, ASOs or other modalities.
MY TOP 5 INSIGHTS FROM THE AMERICAN EPILEPSY SOCIETY MEETING
In one line: AES 2019 was the year of genetic therapies for the developmental and epileptic encephalopathies.
In more detail, here is the list of what I found the most interesting at the AES 2019 meeting:
1. BEYOND SYMPTOMS: GENETIC THERAPIES
In the recent years, many large efforts in understanding the genetic basis of epilepsies led to the identification of hundreds of genes causing developmental and epileptic encephalopathies as well as other epilepsies.
Stoke Therapeutics has a slide in their investors presentation with very impressive numbers. It says:
· 50 million people globally affected by epilepsy
· 50% of epilepsies have an identified genetic cause
· More than 180 disease-associated genes
· 0 genetically-targeted therapies for epilepsies
Their Dravet syndrome program is one of the most advanced therapies that will hopefully change that number zero. It is unbelievable that with hundreds of genetic causes, often leading to severe neurodevelopmental syndromes with aggressive epilepsy and high unmet need, we have so far not one single therapy that corrects the genetic defect or its direct consequences. And we don’t need to get to the extreme of correcting genes, for some of these epilepsies it should be possible to develop small molecules that will target the disease-causing protein, either with activators or with inhibitors. Cystic Fibrosis has shown us how to do this with CFTR, and there are currently several small molecule approaches in development for the epilepsies aiming to correct the protein dysfunction that results from genetic mutations.
Looking only at corporate programs (not even including academic efforts), and likely missing some projects, the following treatments are in development (thank you to Steve Petrou for summarizing many in his slides!):
Dravet syndrome caused by SCN1A mutations: SCN1A upregulation from Stoke (ASO) and Encoded Therapeutics (AAV), and early stage ion channel activators from Xenon and Lundbeck (small molecules)
SCN2A-associated conditions: SCN2A downregulation from RogCon/Ionis (ASO), ion channel inhibitor from Xenon and Praxis (small molecule)
SCN8A developmental epileptic encephalopathy: ion channel inhibitor from Xenon (small molecule)
KCNQ2 epileptic encephalopathy: ion channel modulator from Xenon and Knopp (small molecules)
KCNT1 epileptic encephalopathy: KCNT1 downregulation from Ionis (ASO)
CDKL5 deficiency disorder: enzyme replacement therapy from Amicus (biologic) and gene therapy from Amicus and Ultragenyx (AAV).
Angelman syndrome: activation of paternal UBE3A from Ionis, Roche and Ultragenyx (ASO), and gene therapy from PTC and Pfizer (AAV)
The list is not complete, these are just some of the programs I know about or that were presented at AES. We also saw at AES some early data on the feasibility of using the Stoke platform to upregulate SynGAP1, so it is clear that the current technologies will be able to target many more of the developmental and epileptic encephalopathies, and the significant corporate activity in the space is a very good sign. And there are also many academic programs developing genetic therapies that might lead to clinical programs in the next few years.
This is just the beginning.
2. BEYOND SEIZURES
One aspect I particularly liked was to hear more and more neurologists make a difference between the symptom (seizures) and the condition (epilepsy).
Until recently it was common use “epilepsy” as meaning just seizures, and to hear “epilepsy comorbidities” to refer to the non-seizure epilepsy-associated aspects of the disease such as depression, cognitive delay or motor problems. Calling them comorbid hinted a secondary, less important, role in the disease.
In the last two years I hear clinicians speak more about epilepsy as inclusive of both seizures and the non-seizure aspects. By expanding the definition of epilepsy to go beyond seizures, we open the door for the development of new therapies that also go beyond seizure treatment. There is more and more research around those other aspects of the disease, which are very common in the developmental and epileptic encephalopathies, and the term anti-epileptic medications, referring to the classical drugs, is being replaced by anti-seizure medications. This is not just a linguistic shift, this is a broadening of the scope of a medical and research specialty, to the benefit of patients.
3. AND NOW WHAT? THE CHALLENGE OF CLINICAL TRIALS BEYOND SEIZURES
The good news is that we now understand that epilepsy conditions go well-beyond seizures and start having the therapies that could address the different disease aspects.
The bad news is that no one really knows how to design those “beyond seizures” trials, or what regulators will want to see from those therapies.
This is right now a space of enormous activity. We saw presentations at AES 2019 from companies and academic groups on what to measure in clinical trials for some of these disorders, and whenever I sit down with a patient organization to learn about clinical trials in the space this is a the top of everybody’s list: how to design trials to show efficacy beyond seizure reduction, or to show disease-modification (changes in the course of the disease). We don’t have the answers yet, this is work in progress, but we are all figuring this out together and I was happy to see the challenge of designing these trials also being discussed at AES.
4. OPENING THE COMMUNITY
Everyone who has come to the AES meetings knows that there is a very active patient community. This is precisely because of the developmental and epileptic encephalopathies, which are rare diseases (affecting less than 200,000 people in America), and the rare disease space has a very strong patient advocacy component. As a result, there is a growing number of satellite patient-scientist meetings during the AES congress and you can see patients (often parents) walking around the exhibition hall meeting companies, participating in the discussions and even presenting science.
All of this is great, but I want to put forward two ideas to make this better, since the patient groups are an essential part of the epilepsy community:
1. We need to make it easier for patient groups to attend AES. I have gone to many conferences representing a patient group and it is just too expensive to attend when using personal or charity funding. So we see things like only registering some attendees and swapping badges at the entry, or even not registering at all and just scheduling a bunch of meetings with companies and clinicians at the lobby of the nearest hotel. We all know this. And we should not ignore it. I propose sponsoring patient groups attendance to the major medical conferences in their field, with free registration (at a minimum) and possibly travel support sponsored by companies but managed by the conference organization. I don’t need to see floor stickers on the nearest hotel from (and paid by) a pharma company; I want to see those necessary members of the community being properly invited (and supported) to be in the room instead.
2. We need to make it easier to schedule the patient-run symposiums or round tables at the meeting. Right now these are not official part of the AES meeting so the different groups try to make it convenient to attendees by scheduling their meetings early in the day, or the day before the AES conference starts, and hosting them nearby the conference center. Often they clash (there not that many early mornings!) so many professionals who work on multiple syndromes have to choose which one to attend. I propose protecting some time before the conference for hosting these patient-led meetings. We have traditionally had the Dravet Syndrome Foundation Research Roundtable the day before AES in the evening. The main AES program starts the following day in the afternoon, and many people interested in Dravet knows that they should get to the hosting city one day before so they start with the Dravet Roundtable. That works great. I could see a program where we have a first day that is devoted to patient-organized symposiums, followed by the 4-5 days of main conference agenda. We can’t keep ignoring that the patient organizations are not only an important part of the attendees, but also contribute to part of the agenda. This is already happening. We must get them into the actual agenda as much as possible.
I would love to know what you think about these.
5. GENETIC TESTING IS URGENT
Genetic testing of people with epilepsy is not a “nice to have” anymore. With the discovery of hundreds of genes, and now with the development of genetic therapies, we need to know who those patients are (in addition to the many other values of knowing the cause of your disease). BioMarin, Stoke and Xenon sponsor a free (sponsored) genetic program at Invitae that will soon be open to all children in the US and Canada 8 years and younger. Currently it is for age 5 and younger. This is an excellent model and perhaps the most effective way of making sure that children with epilepsy get early genetic testing. I thank and congratulate the for companies for offering this program.
As a next step, I would like to see this expanded beyond north America (likely through other providers) and also into broader ages. I hope the data obtained by Invitae so far will help document the return on investment for these sponsored programs, and encourage their expansion.
LOOKING INTO 2020
I am excited to see the genetic therapies trials in 2020. From ion channel modulators that might help children with devastating syndromes to the arrival of the first genetic treatment for Dravet syndrome to clinical trials.
Also, the NINDS will host a very special epilepsy meeting called Curing the Epilepsies 2020: Setting Research Priorities in April 2020. This meeting follows one in 2013, and will help establish the NINDS Benchmarks for Epilepsy Research (see here the 2014 ones). The Benchmarks help shape the research priorities from NINDS on epilepsy for the next 7 years so these meetings are extremely important. I am trilled to be a member of the joint AES-NINDS team putting together the next edition of the benchmarks, and encourage you all to send your ideas before February 20 2020 though this link. This only happens every 7 years so don’t miss the chance to shape those priorities!
And I also look forward to more progresses in more developmental and epileptic encephalopathies next year. For example, I know there is a lot more going on in CDKL5 Deficiency Disorder than what we saw at AES, and I hope there will more presented next year.
Above all, 2020 will be the year of clinical trials with genetic therapies in development and epileptic encephalopathies. And I can’t wait!
Ana Mingorance, PhD
Repaso del Foro CDKL5 2019
La quinta edición del Foro CDKL5 tuvo lugar en Boston, los días 4 y 5 de noviembre. El Foro es una reunión anual exclusivamente por invitación que organiza la Fundación Loulou y en la que científicos y miembros de la industria farmacéutica se reúnen con representantes de la comunidad de pacientes para repasar los últimos avances en el campo. Este ha sido mi tercer Foro CDKL5, y el segundo desde que me uní a la Fundación Loulou.
Este es un repaso para los grupos de pacientes de las principales novedades del Foro CDKL5 2019. [SPANISH VERSION - ALSO AVAILABLE IN ENGLISH]
La quinta edición del Foro CDKL5 tuvo lugar la semana pasada en Boston, los días 4 y 5 de noviembre. El Foro es una reunión anual que organiza la Fundación Loulou y en la que científicos y miembros de la industria farmacéutica se reúnen con representantes de la comunidad de pacientes para repasar los últimos avances en el campo. Este ha sido mi tercer Foro CDKL5, y el segundo desde que me uní a la Fundación Loulou.
Al ser la quinta edición, el Director del Foro CDKL5 y querido amigo mío Dan Lavery ofreció un repaso de lo mucho que ha cambiado el campo desde la primera edición del Foro. ¡y ha cambiado tanto que parece que hablemos de dos enfermedades diferentes!
Así que voy a tomar prestada la revisión de Dan de “entonces versus ahora” para compartir con vosotros un repaso del último Foro CDKL5 y de cuanto ha cambiado la investigación y desarrollo de terapias para la deficiencia en CDKL5.
1. DE ENFERMEDAD ULTRA-RARA A SER UNA DE LAS CAUSAS GENÉTICAS DE EPILEPSIA MAS COMUNES
Cuando se celebró el primer Foro en 2015, se pensaba que el síndrome de deficiencia en CDKL5 (CDD por sus siglas en inglés) afectaba en torno a 200 personas en todo el mundo. Pronto nos dimos cuenta de que debía haber muchos más, pero la cifra concreta de casos ha sido muy difícil de calcular.
Este año por primera vez se publicó un estudio de incidencia de CDD, y ahora sabemos que nace un niño con CDD en cada 42.400 nacimientos. Es estudio se hizo siguiendo a todos los nacidos en Escocia durante tres años, que son más de 150.000 nacimientos, y haciendo un test genético completo a todos los niños que presentaron epilepsia antes de su tercer cumpleaños. Esta metodología hace que los resultados sean muy sólidos, y apunta a que el gen CDKL5 es una de las causas genéticas más comunes de epilepsia. Así que ahora sabemos que la cifra real no es de 200 casos, sino de más de 20.000 casos en todo el mundo. ¡para nada una enfermedad ultra-rara!
La cifra que todavía no sabemos es la prevalencia, que es el número total de pacientes diagnosticados. Esto se debe a dos razones. La primera es que como los tests genéticos solo se están haciendo en los últimos años, nos faltan por diagnosticar la mayoría de los pacientes adultos. Y la segunda es que no tenemos una forma buena de localizar a todos los casos diagnosticados para poder contarlos.
Para intentar encontrar todos estos casos, desde la Fundción Loulou y el grupo de pacientes americanos IFCR pedimos este año la creación de un código ICD-10 para el síndrome. Estos son los códigos que usan los médicos cuando diagnostican un paciente, y el tener un código nos permitirá hacer estudios epidemiológicos ya que habrá una forma de identificar todos los casos. Entre tanto, una cosa está bien clara: hablamos de buscar decenas de miles de pacientes, no unos pocos cientos.
2. DE QUINASA HUÉRFANA A REGULADOR GENERAL
Si hay un área en la que el campo de CDKL5 ha avanzado de forma brutal desde 2015 es en la comprensión de qué hace CDKL5 en el cerebro. Estaba claro que se trataba de una proteína quinasa, las que activan e inactivan otras proteínas como si fueran el interruptor de la luz, pero la identidad de esas proteínas concretas que se encienden y se apagan controladas por CDKL5, y lo que estas hacen en las neuronas, eso era desconocido.
Ahora en 2019 los científicos han identificado muchas de estas proteínas controladas por CDKL5, y han desarrollado reactivos de laboratorio (anticuerpos) para poder ver donde y cuando CDKL5 está activa en el cerebro. Los científicos han averiguado también que CDKL5 controla muchas proteínas asociadas con el esqueleto neuronal (el citoesqueleto) y que es muy posible que es a través de este proceso que CDKL5 controla la presencia de ciertos receptores en la superficie de las neuronas. Como resultado de todo esto, la falta de CDKL5 lleva a que las sinapsis (las conexiones neuronales) permanezcan más inmaduras, y que tengan en su superficie receptores típicos de neuronas más inmaduras, y eso lleva a hiperexcitabilidad neuronal.
Saber todo esto tiene dos implicaciones importantes de cara a desarrollar terapias para la enfermedad. La primera es que como CDKL5 controla tantos procesos celulares, va a ser muy difícil de compensar su falta actuando sobre otras partes de la célula. Está claro que hace falta volver a poner CDKl5, reemplazando el gen o la proteína. Las buenas noticias es que este tipo de tratamiento está ya en desarrollo.
La segunda implicación importante es que lo que no hemos visto es falta de conexiones entre partes del cerebro, cambios de estructura cerebral, o muerte neuronal o cualquier otro proceso neurodegenerativo. Por lo tanto parece que CDKL5 hace falta todo el tiempo para mantener el proceso constante de formación de conexiones entre neuronas (sinapsis). Y esto indica que si podemos devolver CDKl5 al cerebro tenemos muchas posibilidades de obtener eficacia incluso en cerebros maduros. Eso no sería así si CDKL5 hiciera falta para la migración neuronal (que pasa en bebés un niños muy pequeños), o si la falta de CDKL5 llevara a muerte neuronal. En esos casos solo tendríamos una ventana temporal para actuar muy limitada. Pero basado en lo que sabemos ya de la biología de CDKL5, personalmente creo que CDD es una enfermedad candidata para terapia génica o de reemplazo enzimático, incluso en adultos.
3. DE CERO A CUATRO ENSAYOS CLÍNICOS, Y MÁS EN CAMINO
Otro aspecto que ha cambiado dramáticamente en CDD desde 2015 es el interés de la industria farmacéutica en la enfermedad. La primera empresa en tomar el testigo fue Marinus, y anunciaron este Foro que están cerca de cerrar el reclutamiento de su ensayo clínico cumpliendo los plazos planeados. Y estamos hablando de 100 pacientes.
Pensad en la cifra: en a penas un par de años hemos pasado de pensar que no hay más que 200 pacientes en todo el mundo, a ser capaz de hacer ensayos clínicos de 100 pacientes a la vez que hay otros ensayos en paralelo.
Porque Marinus no está solo, hay tres ensayos clínicos adicionales en CDD ahora mismo, todos en fase 2. PTC acaba de completar el ensayo con ataluren en pacientes con CDD debido a mutaciones non-sense y están analizando los datos. Ovid y Takeda tienen un ensayo en marcha con el fármaco TAK-935. Y la universidad de Nueva York está realizando un ensayos clínico con fenfluramina, el fármaco de Zogenix para el síndrome de Dravet. Durante el Foro, Zogenix recibió el Premio de Excelencia del Foro CDKL5 por su contribución clínica a través de este ensayo en CDD.
Así que hemos pasado de tener una enfermedad que se pensaba que era ultra-rara, y que no estaba ni reconocida como enfermedad independiente por los reguladores porque incluso la comunidad médica la confundía con Rett, a hablar de una enfermedad única claramente independiente que recibe designaciones de fármaco huérfano y que tiene cuatro ensayos clínicos por cinco empresas farmacéuticas (Takeda y Ovid trabajan juntas en su programa para CDD).
Y estas cinco empresas tampoco están solas, empresas como Amicus y Ultragenyx que estaban presentes en el Foro CDKL5 están trabajando en terapias curativas para CDD, y pudimos ver varias terapias adicionales durante el Foro:
Una colaboración entre el Trinity College de Dublín y la Universidad de Insubria nos mostró como un fármaco que modula el esqueleto neuronal (que no funciona bien cuando falta CDKL5) mejora varias de los problemas neurológicos en ratones con CDD.
Un grupo de la Universidad de Pensilvania nos enseñó como otro fármaco, que actúa solo en receptores típicos de neuronas immaduras pero que en los cerebros con CDD siguen estando presentes mucho mas tarde, también mejora varias de los problemas neurológicos en ratones con CDD.
Este mismo grupo también nos enseñó como en ratones con CDD, el cannabidiol corrige también varios problemas neurológicos, lo que cuadra con datos de GW Pharma de que Epidyolex podría tener eficacia en esta enfermedad.
E incluso la empresa Takeda nos presentó resultados con un segundo fármaco (no el que tienen en ensayos clínicos) y que también corrige algunos de los problemas neurológicos en ratones con CDD actuando sobre otras proteínas en las neuronas. Incluso han obtenido la designación de fármaco huérfano para este posible tratamiento para CDD por la agencia americana del medicamento (la FDA) hace unos meses.
Como veis cuatro ensayos en ratones que nos muestran que hay muchas más terapias en desarrollo y que podrían avanzar hacia ensayos clínicos, y que ayudarían a controlar no solo la epilepsia sino también otros de los problemas asociados con CDD. Y estos son solo algunos de los futuros tratamientos que vimos, ahora os cuento algunos más en las sesiones siguientes.
Lo que es importante es que sepáis que todas estas posibilidades nos llegan por lo mucho que hemos avanzado en nuestra comprensión de las funciones de CDKl5 en las neuronas en situaciones normales, de lo que pasa cuando la proteína falta, y de como la enfermedad se presenta en ratones cuando les falta CDKL5 (que no son los mismos síntomas exactamente que en personas). Y esto ha sido posible gracias al trabajo de los grupos de pacientes y la Fundación Loulou. Esta es una de las formas en los que los grupos de pacientes pueden tener un gran impacto, financiando los proyectos tempranos y la creación de modelos animales que su vez permiten que los grupos de investigación puedan más adelante asegurar financiación pública que suele ser tremendamente competitiva. Dar un impulso inicial para que el campo luego pueda despegar solo.
4. DE SÍNTOMAS A CURAS
Tanto los cuatro tratamientos con datos en ratones que os acabo de describir como los ensayos clínicos en marcha (salvo por ataluren), ayudan a que el cerebro funcione mejor pero ni corrigen la mutación en el gen CDKL5 ni la falta de la proteína CDKL5 en el cerebro. Ayudan a que el cerebro funcione mejor sin necesitarlos.
Tengo una diapo que uso en conferencias con familias donde explico los distintos tipos de terapias que pueden ser desarrolladas para CDD a diferentes niveles. Y la diapo es perfecta para explicar esto. Esencialmente sabemos que en CDD el cerebro no funciona bien, porque la falta la proteína CDKL5, porque tiene una mutación en el gen CDKL5. Y sabiendo esto podemos pensar en tratamientos a diferentes niveles. Por ejemplo podemos pensar en tratamientos que hagan que el cerebro funcione mejor a pesar de no tener CDKl5. O en volver a poner la proteína. O en volver a poner el gen. O en tratamientos mas complicados que puedan corregir la mutación del gen, o quizás reactivar la segunda copia del gen en el segundo cromosoma X y que no está siendo usada.
En las primeras secciones describía los progresos que han tenido lugar en el desarrollo de terapias capaces de hacer que el cerebro funcione mejor a pesar de no tener CDKL5. Pero donde hemos visto una explosión enorme en la ciencia ha sido en las otras estrategias: las que corrigen la causa de la enfermedad.
En una de las sesiones en el Foro teníamos un equipazo impresionante sobre el pódium: Kyle Fink de la Universidad de California Davis, Jim Wilson de la Universidad de Pensilvania, y David Liu del Broad Institute en Boston. Uno a uno nos enseñaron tres formas diferentes de hacer que las neuronas puedan producir CDKL5, y parecía que estuviéramos viendo la conferencia en un futuro que cada vez está mas cercano, cuando la ciencia pueda corregir las mutaciones que causan enfermedades genéticas.
Primero Jim Wilson nos enseño sus resultados con un proyecto de terapia génica que están llevando a cabo en Filadelfia y en el que están desarrollando un virus al que le quitan todo el ADN del virus y en su lugar le ponen el gen CDKL5 humano. Cuando inyectan estos virus en el cerebro de ratones con CDD, empiezan a producir CDKL5 y les consiguen mejorar muchos de los problemas neurológicos. Jim explicó que aún quedan algunos pasos hasta poder hablar de ensayos clínicos, como saber cuanto CDKL5 hace falta añadir, y en qué células en concreto, y cómo de seguro es todo el proceso antes de poder empezar ensayos. Pero como yo lo veo, viendo todo lo que tienen ya avanzado, creo que estamos hablando de un par de años para que la terapia génica legue a ensayos para CDD, y eso en cuanto a plazos de desarrollar nuevas medicinas es básicamente estar a la vuelta de la esquina.
Hay que recordar que dos empresas, Amicus y Ultragenyx, también están trabajando en desarrollar terapias génicas para CDD (aunque no presentaron su investigación en el Foro). Incluso Ultragenyx se llevó este año el Premio de Excelencia del Foro CDKL5 por su contribución preclínica (trabajo en animales previo a ensayos). Con tantos esfuerzos paralelos en marcha confío en que uno o mas de esos programas lleguen a ensayos clínicos.
Luego Kyle Fink nos enseñó los resultados de un proyecto que están llevando a cabo para reactivar la segunda copia del gen CDKL5 que tenemos las mujeres. Como las células masculinas solo tienen un cromosoma X (son XY), las células femeninas (XX) inactivan una de las dos copias para así no producir el doble de niveles de todas las proteínas que están codificadas por genes del cromosoma X. Así que en mujeres cada una de nuestras células aleatoriamente inactiva el cromosoma X que tenemos de papá o el que tenemos de mamá. Y lo que pasa cuando en uno de esas dos copias hay un gen con una mutación es que la mitad de nuestras células estará bien, porque por suerte inactivaron el cromosoma que llevaba la copia mala del gen, pero la otra mitad tendrá deficiencia en CDKL5 porque inactivaron el cromosoma que lleva la copia buena y solo tienen disponible la copia mutada. Lo que el laboratorio de Kyle busca es desarrollar herramientas basadas en la teconolgía de CRISPR que les permitan localizar el CDKL5 inactivo en el cromosoma X que no se está expresando, y rescatarlo sin tocar ninguno de los otros genes que hay alrededor. Es la leche lo que intentan, y hasta hace poco parecía de ciencia ficción. Pero ya lo están haciendo con éxito en células en cultivo (todavía no en ratones con CDD) y consiguen que las células lean las dos copias del gen CDKL5, con lo que si una de las copias está mutada las neuronas tendrán justo lo que necesitan: una copia funcional del gen en cada célula. El paso siguiente es desarrollar la forma de poder poner en el cerebro esos reactivos tipo CRISPR que están usando (posiblemente metiéndolos en virus, como en la terapia génica) y probarlos en ratones.
Y por último David Liu presentó la última frontera de la edición genética: el prime editing. Edición genética es cuando puedes corregir la mutación en el gen. No es poderle a la célula un gen nuevo que le lleva en un virus. No es activar la copia del gen que tenia la célula escondida en el segundo cromosoma X. No. Es llegar a la mutación y arreglarla.
Esta estrategia es la que recientemente publicaron en la revista Nature, y que salió en las noticias de todo el mundo. Lo que el laboratorio de David consigue hacer con su última versión de prime editing es lo que hasta ahora parecía imposible arreglar: los casos en los que al niño le faltan una o dos letras del gen, o se le han insertado una o dos letras en el gen (mutaciones de pérdida de pauta de lectura). Porque ya sabíamos que CRISPR te puede cambiar una letra por otra, darte el cambiazo entre la letra mala y la buena, pero no podía poner o quitar letras. Y ahora David Liu si que puede, y nos enseñó como están empezando a aplicar esta tecnología a células en cultivo con mutaciones de este tipo de CDKL5. Como os decía con el proyecto de reactivación del cromosoma X, lo siguiente sería buscar cómo hacer llegar hasta el cerebro esos reactivos tipo CRISPR que están usando (posiblemente metiéndolos en virus, como en la terapia génica) y probarlos en ratones.
Como estos dos últimos proyectos están en estadíos mas tempranos, yo les calculo más años para llegar a ensayos clínicos que la terapia génica “clásica” de usar un virus con el gen CDKL5. Pero saber que es factible hacer estos cambios en las células, y saber que lo están aplicando ya a CDD, es alucinante y una muy buena noticia para todos nosotros.
Y luego habló el hombre que busca tumbar todos estos plazos, y que nos invitó a todos en el Foro a pensar y trabajar de forma diferente. Se trata de Tim Yu, de Harvard y el hospital infantil de Boston. En el mundo de las enfermedades raras casi todos hemos oído hablar de Mila, la niña con la enfermedad de Batten para que desarrollaron una terapia personalizada en tiempo record para intentar frenar la progresión de su terrible enfermedad. La terapia no es como un fármaco tradicional, sino que se parece mas a un cachito de ADN (oligonucleótido se llama), y lo llamaron Milasen por Mila. Y ese científico que fue capaz de desarrollar Milasen para poder tratar a Mila en tan solo 12 meses desde su diagnóstico es Tim Yu. Al igual que David Liu con prime editing, el desarrollo de Milasen también salió en todas las noticias recientemente. Aprendimos de Tim que no todas las mutaciones son candidatas a este tipo de terapia personalizada con un cacho de ADN, y son sobre todo las que causan defectos de “splicing” en el gen que son candidatas (no muchas de las missense, nonsense o de cambio de pauta de lectura, o cuando falta un cacho del gen). Pero para los casos en los que la mutación es candidata, el laboratorio de Yu está buscando tumbar los plazos tradicionales de desarrollo de medicamentos y poder no ya curar enfermedades de una en una, sino personas con medicina personalizada de una en una (ya que la terapia solo sirve para uno). Esto es una revolución para la medicina, y tenemos suerte de vivir en este momento de la historia en la que estos avances están cambiando la forma en la que practicamos la medicina.
Por cierto que se que muchas familias os liais con saber qué tipo de terapia servirá para todos o qué tipo de terapia es solo para cierto grupo de pacientes. Os he preparado un cuadro de texto con aclaraciones para CDD.
En breve: hemos pasado de decir “cómo podemos hacer que las empresas que trabajan en epilepsia se interesen por CDD” a decir “como podemos tener reemplazo enzimático, Y TAMBIEN terapia génica, Y TAMBIEN reactivación del gen en el cromosoma X, Y TAMBIEN edición genética” para CDD. Y todo en menos de 5 años. Es increíble.
5. DE PRECLÍNICO A CLÍNICO
Una consecuencia de la explosión en la investigación en torno a CDD es que los proyectos han ido madurando y la cartera de programas que se acercan a ensayos clínicos (o que ya están en ensayos) crece a gran velocidad. Esto implica que en las fundaciones y grupos de pacientes nos hemos tenido que poner las pilas y prepararnos para los ensayos.
En la Fundación Loulou hemos estados muy liados este año solicitando la inclusión de CDD en las clasificaciones médicas, apoyando a las empresas que están hablando con agencias reguladoras del medicamento para que tengan toda la información que necesitan, preparando un modelo conceptual de la enfermedad (una cosa un poco técnico de explicar pero que es importante para los ensayos), haciendo estudios sobre medidas que se puedan usar en ensayos clínicos, hablando con empresas farmacéuticas de la posibilidad de que trabajen juntas en solventar los desafíos de los ensayos clínicos en vez de cada uno por su parte, en hacer reuniones con familias para entender mejor lo que valorarían de nuevos medicamentos, e incluso hicimos una reunión con la agencia americana del medicamento (ver mas abajo).
¡No es que hayamos dejado de trabajar en la biología y la investigación en animales! Lo que hemos hecho ha sido expandir el rango de disciplinas en las que trabajamos. En el Foro CDKL5 hicimos 8 reuniones paralelas para tener grupos pequeños de científicos, gente de industria, médicos y pacientes, trabajar en cada uno de los 8 eslabones en la cadena de desarrollos de fármacos, cada uno centrado en un eslabón desde entender que le pasa a las células sin CDKL5 hasta terminar ensayos y pedir la aprobación de un fármaco. Hemos avanzado mucho en cada uno de estos eslabones pero queda trabajo, y para eso estas 8 reuniones paralelas, para identificar lo que falta y los esfuerzos que requerirá.
El que ahora trabajemos mucho mas en las etapas clínicas de desarrollo de fármacos es reflejo de lo mucho que ha avanzado y madurado el campo. Hemos crecido mucho, en tiempo record.
6. LA VOZ DEL PACIENTE, ALTA Y CLARA
Por último, ya que hablamos de haber crecido mucho, debo comentar en el crecimiento de la comunidad de pacientes en CDD. Hemos pasado de arrancar con unas pocas familias, sobre todo en EEUU, Inglaterra e Italia, a tener ahora la Alianza Internacional CDKL5, y organizaciones de pacientes en 18 países incluidos Brasil, China y Japón.
La Alianza organizó su congreso anual en junio, organizado por CDKL5 Inglaterra, y os recomiendo leer sobre este congreso. Conozco muchas comunidades de pacientes y esta es excepcional por lo bien que funciona y lo unidos que están los grupos.
Durante el Foro CDKL5 de este año, miembros de la Alianza actuaron como co-moderadores de esas 8 reuniones paralelas, junto con un moderador de la industria o médicos. Y esto no es algo que se vea mucho, un congreso en el que la comunidad de pacientes esté tan integrada en todas las conversaciones a todos los niveles de la cadena de desarrollo de fármacos, de células a ratones a biomarcadores a ensayos. No tengo duda de que la fuerza de esta comunidad de pacientes es una de las armas secretas del campo de CDKL5.
Y esa fuerza quedó particularmente evidente el 1 de noviembre cuando la comunidad de pacientes de estados unidos se reunió con la agencia americana del medicamento (la FDA) en un Patient-Focused Drug Discovery meeting organizado entre el grupo de pacientes americano y la Loulou Foundation. Un Patient-Focused Drug Discovery meeting (PFDD), o reunión de desarrollo de fármacos centrada en el paciente, son reuniones en las que la comunidad de pacientes se reúne con la FDA para hablar de la enfermedad, y en la que son las familias las que hablan y los reguladores los que escuchan. Este fue sin duda uno de los grandes logros de este año, y podeis ver el video donde los 10 panelistas (padres y abuelos de niñ@s con CDD) y los participantes en la sala explican cómo es vivir día a día con CDD, en que medida impacta a toda la familia, y lo que esperan de futuros tratamientos.
Ya dedicaré una entrada separada al PFDD. Entre tanto quiero destacar que teniendo al menos 7 mil enfermedades raras en el mundo, mas todas las no raras, somos tan solo la enfermedad numero 32 que ha conseguido celebrar este tipo de reunión con la FDA. Y esto refleja la velocidad de desarrollo de terapias para CDD, que ha forzado el interés por la reunión, y la fuerza de esta comunidad de pacientes.
Cuando nació la Fundación Loulou, los padres de Loulou se fijaron como una meta llegar a tener tratamientos (al menos en ensayos) para 2020, y curas para el 2025. Ahora ya sabemos que en 2020 Marinus terminará el ensayo de fase 3 con ganaxolona, y que habremos tenido como mínimo 4 ensayos clínicos en CDD, y colectivamente tratado a más de 150 personas con CDD que podrán beneficiarse ya de estos tratamientos. Y personalmente creo que para 2025 tendremos varias terapias curativas (como la terapia génica) en ensayos clínicos avanzados, y quizás alguna aprobada.
SI puedes imaginarlo, puedes conseguirlo.
Espero que os haya gustado el resumen. Ya me diréis lo que os parece en los comentarios. Y os dejo también el enlace al resumen del Foro de 2018 (en inglés).
Ana Mingorance PhD
Nota: este texto captura mis impresiones de las presentaciones del Foro que más me interesaron como científico y como defensora de los pacientes, no es un texto oficial del congreso emitido por la Fundación Loulou. Escribo estos resúmenes para los padres de personas con CDD, así que a veces me tomo ciertas licencias a la hora de explicar las partes mas técnicas ;-)
MAIN LESSONS FROM THE 2019 CDKL5 FORUM
For the past five years the Loulou Foundation hosts an annual meeting where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances. This was the third Forum I attended, and my second since joining the Loulou Foundation.
Here are the main news and take-home messages from the 2019 CDKL5 Forum that took place in Boston in November 4 and 5.
Versión en Español en este enlace.
The 5th edition of the CDKL5 Forum recently took place in Boston, in November 4 and 5. The Forum is an annual meeting hosted by the Loulou Foundation where scientists and drug developers working on CDKL5 deficiency, together with representatives from patient organizations, meet to discuss the latest advances. This was the third Forum I attended, and the second since joining the Loulou Foundation.
Because of the 5th anniversary, the CDKL5 Forum Director (and my dear friend) Dan Lavery offered an update of how much the entire CDKL5 deficiency field has changed since the first Forum edition. And it has changed so much that it seems we are looking at two different diseases!
So I will borrow Dan’s review of then versus now to share with you an update about the recent CDKL5 Forum and highlight how far along we have come in the CDKL5 Deficiency Disorder field.
1. FROM ULTRA-RARE DISEASE, TO BEING ONE OF THE MOST COMMON GENETIC CAUSES OF EPILEPSY
When the first Forum took place in 2015, CDKL5 Deficiency Disorder (CDD) was thought to affect maybe around 200 children worldwide. It would soon become clear that there were many more cases, but having a specific number has remained a challenge.
This year we obtained the first well-documented incidence estimate for CDD, with mutations in CDKL5 being found in 1 out of every 42,400 live births. The study followed a cohort of all children born in Scotland over 3 years (over 150,000 births), and genotyped all those having epilepsy during the first 3 years of their life. This methodology makes the study very solid, and indicates that CDKL5 would be one of the most common genetic causes of epilepsy in children. Now we know that the real numbers are not 200 cases, but well over 20,000 cases. Definitely not an ultra-rare disease!
The real number of patients diagnosed (prevalence) is still unclear. This is because of two reasons. One, because genetic testing is only regularly done in the recent years, so most adult patients remain undiagnosed even though they exist out there. And two, because we don’t have a good way to track the number of patient diagnosed.
To address this, the Loulou Foundation and IFCR applied this year for CDD to have a unique ICD-10 code, which will allow clinicians to use this diagnostic code with their patients and support the epidemiological studies that are needed. In the meantime, one thing is clear: we are looking for tens of thousands of patients out there, not hundreds.
2. FROM ORPHAN KINASE TO MASTER REGULATOR
If there is one area where the field has evolved dramatically since 2015 is the understanding of what CDKL5 does in the brain. It was clear that it was a kinase, those proteins that turn other proteins on and off as if they had a light switch, but the specific proteins that get turn on or off by CDKL5, and what these do in the neurons, was unknown.
Now scientists have identified many of the target proteins of CDKL5, and produced lab tools (antibodies) that allow us to see when and where CDKL5 is active in the brain. Scientists have also identified that CDKL5 controls a number of proteins associated with the neuronal skeleton (the cytoskeleton) and it is likely through this process that it regulates the presence at the membrane of different proteins including receptors. As a result of this, in the absence of CDKL5 , the formation of synapses (neuronal connections) will remain in more immature stages and the presence of receptors will also resemble the most immature brain state, leading to neuronal hyperexcitability.
There are two important implications for therapies in these results. The first one is that with CDKL5 controlling so many processes it will be hard to “bypass it” or achieve a full compensatory benefit with treatments that target other pathways. We really should try to replace the protein or the gene to achieve the full recovery. The good news is that these treatments are all in development.
And the second important implication is that we have not found large changes in neuronal wiring, or in brain anatomy, and we have certainly not seen neuronal loss or any sign of neurodegeneration. So it would appear that CDKL5 is constantly needed for the very dynamic process of synaptic plasticity and formation. This means that bringing CDKL5 expression back is likely to provide a benefit even in the more mature brains, while in diseases that affect neuronal migration, or that lead to neuronal death, you only have a narrow time window to replace the protein and see any improvement. Based on the biology that we know, I believe CDD will be a good disease for gene therapy or enzyme replacement therapy even in adult patients.
3. FROM ZERO TO FOUR CLINICAL TRIALS AND A GROWING PIPELINE
Another area that has changed dramatically since 2015 is the corporate interest in CDD for the development of treatments. The company who first took the lead was Marinus, and they announced during the Forum that they are on track to soon close their target recruitment for the ongoing Phase 3 trial in CDD, which is 100 patients.
Think about it, we have gone from thinking that there were only 200 patients in the world to being able to run 100-patient trials in parallel to other trials in just a few years.
Because the Marinus trial is not alone, there are three additional clinical trials in CDD going on right now, all in Phase 2 (pilot) stages. PTC recently completed a trial with ataluren for CDD patients with non-sense mutations in CDKL5 and are analyzing the data. Ovid and Takeda have a clinical trial ongoing where they are enrolling around 15 patients with CDD for treatment with their drug TAK-935. And a 10-patient investigator-initiated study with fenfluramine, developed by Zogenix, is starting in New York. During the Forum, Zogenix received the CDKL5 Forum Award of Excellence for “Company Making a Difference” in the clinical space for supporting the latest clinical study in CDD.
So we have gone from having a disease that was thought to be ultra-rare, and that was not recognized as a stand-alone disease by regulators because even the medical community confused it with Rett syndrome, to having a clearly independent unique disease with orphan drug designations and having four clinical trials by five pharmaceutical companies (TAK-935 is developed by Ovid and Takeda together).
And these five companies are not alone. Many companies in the room such as Amicus and Ultragenyx are developing treatment for putting back CDKL5 into the brain, and we were able to see several potential additional therapies presented during the Forum:
A collaboration between the Trinity College Dublin and Insubria University showed that a drug that acts on the neuronal skeleton (which does not work well in the absence of CDKL5) can correct memory problems in mice with CDD.
A group from the University of Pennsylvania showed that another drug, which acts only in immature neuronal receptors that happen to remain present for too long in brains with CDD, also corrects some of the neurological problems in mice with CDD.
The same group also showed that cannabidiol addresses some of the neurological problems in mice with CDD, supporting the data by GW Pharma that Epidiolex could have efficacy in this disease as well.
And even the company Takeda showed a second drug that they are developing (not the one already in CDD trials) and that can also correct some of the neurological problems in mice with CDD by acting on another signaling pathway. They even obtained the Orphan Drug Designation by the FDA for their drug earlier this year.
In total these were four mouse trials showing that there are many more therapies that could move into clinical trials and help reduce not only epilepsy but also other neurological problems associated with CDD. There were some extra treatments presented, but I will cover them in the next section.
What is important to know is that all of these promising findings have been possible because of so much new understanding that we have of how CDKL5 functions in the cell in normal situations, what happens when it is missing, and how CDD presents in mice when their CDKL5 is removed – since it is a bit different than in people. All the investments in research by several patient groups and the Loulou Foundation have made this work possible, and more recently the labs have started being successful at obtaining R01 funding from the NIH. This is one of the areas where the patient community can make a big difference, by supporting the early research and the generation of animal models for the disease that will later allow those groups to be self-sufficient at obtaining the highly-competitive public grants. De-risk early so that a research field can then take off on its own.
4. FROM SYMPTOMS TO CURES
All of the treatments that I listed above in mouse experiments, as well as the current drugs in clinical trials (except for ataluren), help the brain but do not correct the mutation in the CDKL5 gene or the lack of CDKL5 protein in the brain. They help the brain function better with CDKL5.
I have a slide that I use at patient conferences to explain the different types of therapies that could be developed for CDD. That slide is perfect to explain this. Essentially, we know that in CDD the brain does not function well, because it does not have CDKL5 protein, because the CDKL5 gene is mutated. And knowing this we can think of different levels of treatment. We can think of treatments of help the brain function better despite not having CDKL5. Or we could put back the protein. Or we could add a copy of the gene. Or we could go into more difficult approaches and try to correct the mutated copy, or perhaps reactivate the second copy of CDKL5 gene that all females have in their second X chromosome but that is not being used.
In the previous sections I described all the progresses to develop better therapies able to make the brain function better despite not having CDKL5, but where we have seen an explosion of science is in the next approaches: correcting the cause of the disease.
There was a session during the Forum when we had a dream team on stage: Kyle Fink from UC Davis, James Wilson from the University of Philadelphia, and David Liu from the Broad Institute. One by one, they showed us three ways to try to get neurons to produce functional CDKL5, and it was like seeing a conference from the future, when we will be able to correct genetic mutations in a way that we are just beginning to see.
First Jim Wilson presented data from a gene therapy program that he is running at Penn where he has developed a virus that contains the entire CDKL5 gene instead of the virus DNA. When he then injects these virus into the brain of mice with CDD, he can see that the CDKL5 protein is being produced and that this gene therapy corrects some of the neurological problems in mice with CDD. Jim explained that there are still some steps to do before starting clinical trials, like knowing how much CDKL5 is needed, and in which cells, and how safe the entire approach is before it can move into the clinic. But as I see it looks like we are just talking about a couple of years for CDKL5 gene therapy to be ready for trials, and this for medicine is essentially just around the corner.
It is important to know that two companies, Amicus and Ultragenix, are also working very hard for developing gene therapies for CDD (even though they did not present data at the Forum) and that in fact Ultragenyx received the CDKL5 Forum Award of Excellence for “Company Making a Difference” in the preclinical space for their gene therapy program. With so many options ongoing, I trust that one or more of these programs will reach clinical trials.
Then Kyle Fink showed us some data of a project that they are running to rescue the second CDKL5 gene copy that all females have. Because male cells only have one X chromosome (XY), female cells (XX) inactivate one X chromosome so that we don’t produce twice the levels of proteins located in those genes as males do. So all of our cells randomly inactive the X chromosome that we got from dad, and others the one that we got from mum. What happens when one of these chromosomes carries a mutation in the CDKL5 gene is that half of our cells will be fine (they inactivated the “bad” chromosome!) but the other half has deficiency in CDKL5 because they happened to inactivate the “good chromosome” and are left with a mutated CDKL5 gene copy. What the Fink lab is doing is to develop tools based on the famous CRISPR to find the inactive CDKL5 gene in the inactive X chromosome and “release it”, without messing up with any of the other genes in the chromosome. This is very cool, and appeared to be impossible to do until recently. They are doing this right now in cells in culture (not yet in mice with CDD), and the result is that all cells will read both copies of CDKL5, one good and one that is not functioning, and that is perfect because that means that they will all have exactly what they need: one copy that works. Next step is to develop a good technique to get those CRISPR-like tools into the brain (likely into a virus as well) and test it in mice.
Last, David Liu presented what we could call the next frontier of gene editing: prime editing. Gene editing is when you can fix the mutated gene. Not add a new one coming in a virus. Not activating the other copy in the inactive chromosome. But get to the mutated one and fix it.
This approach was recently published in Nature and attractive massive media attention across the globe. What the Liu lab can do with this approach and his latest variations is to correct mutations that until now appeared impossible to correct: those where your child has one or two extra letters in their DNA, or is missing one or two letters in the gene (these are called frame-shift mutations). While we knew that CRISPR would replace one letter, swap the mutated letter by the correct one, we could not insert or delete. Now David Liu can do that, and he told us they are starting to apply it to some mutations in CDKL5. Just like with the Fink approach, the next step after being able to fix the gene in cells in culture is to develop a good technique to get the prime editing tools into the brain (likely into a virus as well) and test it in mice.
Because they are more immature, these last two approaches are likely to take several more years to get to the point when they can be tried in clinical trials. But to know that it is biologically feasible to do these things, and that they are applying it to CDD, is already amazing and tremendously encouraging.
And then there is a man who has been challenging all these timelines, and he too gave a presentation at the Forum and invited us to think about this differently. And that was Tim Yu, from Boston Children’s Hospital and Harvard Medical School. In the rare disease space we all know of Mila, the girl with Batten disease that got a personalized drug developed for her in a record time to try to stop the progression of her terrible disease. The drug is not a traditional type of drug, it looks more like a little piece of DNA (called and oligonucleotide), and got the name of Milasen. And the scientist who developed it and was able to treat Mila just 12 months after her diagnosis was Tim Yu. Just like David Liu with prime editing, this has also been all over the news. We learnt from Tim that not all types of mutations can be targeted with these personalized pieces of DNA, it is mainly the ones that cause “splicing” defects in the gene (not most missense, not the nonsense, not the frameshift, not the ones where a piece of the gene is missing). But for those cases where the mutation would be a candidate, his lab is now pushing the traditional steps of developing a drug for a disease at a time and seeing how to develop the drug for one child at a time. This is a complete revolution, and we are lucky to be living this time where all of these discoveries are changing the way we do medicine.
By the way, I know that many patients and parents get lost with so many genetic approaches and which one would help all patients versus which one is patient-specific, so I have made a separate text box to address this question for CDD.
In short: we have gone from saying “how could get one of the many epilepsy companies to try their drugs in CDD” to saying “how can we have enzyme replacement AND gene therapy AND X-chromosome reactivation AND gene editing all developed for CDD”. All in under 5 years.
Pretty unbelievable.
5. FROM PRECLINICAL TO CLINICAL
A clear consequence of the explosion in research around CDD and the growth of the treatment pipeline is that we have gone from focusing on funding preclinical work (understanding the biology of CDKL5, developing animal models) to having to quickly start working hard on getting the clinical work ready. This means getting ready for clinical trials.
At the Loulou Foundation we have been busy requesting the disease registration in different medical classifications, supporting companies with information as they go through the regulatory process, generating a disease concept model (something that is needed for trials), running studies on clinical outcome assessments (those are the things you measure in clinical trials), discussing with pharmaceutical companies the possibility of working all together to solve the clinical trial challenges as opposed to competing, running meetings with the patient community to understand what they value the most in treatments, and we even hosted a meeting with the FDA together with IFCR (see the next section).
We have not stopped working on the biology and preclinical space! Instead we have expanded what we work on. At the Forum we run 8 separate breakout sessions to have small focused groups of scientists, clinicians and patients go through 8 blocks of what we call “the translational toolbox”, each one focusing on a single topic that combined allowed us to see if we have all we need to take a drug all the way from understanding what happens in the cell to completing clinical trials. We have achieved much in all of these steps but there is still much work to do, so those breakout sessions allowed us to map the needs and focus our efforts in the year until the next Forum.
The greater focus and number of efforts on the clinical space in CDD are a reflection of the maturity of the field. We grew up very fast, in a record time.
6. THE VOICE OF THE PATIENT IS LOUD AND CLEAR
Last but not least, if we are to talk about growth, I must commend on the growth of the CDD patient community. We have gone from having few initial families, organized in the US, UK and Italy, to now have a CDKL5 International Alliance, around 18 national patient organizations, and a world-wide spread that includes countries like Brazil, South Asia, Japan and most recently, China.
The Alliance held a meeting in June, hosted by CDKL5 UK, that I wrote about here and I strongly recommend you reading it if you hadn’t. I have seen many patient communities and this one is exceptional in how well it runs and works together.
During the 2019 CDKL5 Forum, members of the Alliance co-chaired each of the 8 parallel breakout sessions working hand in hand with a clinician, industry professional or scientist chair. This is not something I had ever seen in any other research field, a congress where the patient community is integrated into all discussions along the translational chain, from cells to animals to biomarkers to trials to partnerships. I have no doubt that the strength of this patient community is one of the secret weapons of the CDKL5 field.
And that strength was particularly clear in November 1st, when the US patient community met with the FDA and held an externally-led Patient-Focused Drug Discovery (PFDD) meeting co-hosted between the US patient group (IFCR) and us at the Loulou Foundation. These are meetings where the patient community meets with the FDA to discuss one disease, and where patients speak and the regulators listen. This was a major milestone for the entire field, and I encourage you to watch the video and see the amazing 10 patient (and grandparent!) panelists and the discussions in the room, as the caregiver community explains the regulators how it is to live with CDD, how it impacts the entire family, and the treatment of treatments that they would value the most.
I will dedicate an entire new entry to the PFDD. I just want to highlight here that from all diseases known to mankind, and there are over 7,000 rare diseases alone plus all the non-rare ones, we were the #32 disease-focused PFDD meeting that the FDA had. And that is a reflection of both the fast-growing efforts of the industry to develop treatments for CDD, and the strength of the CDD patient community.
When the Loulou Foundation was born, Loulou’s parents set the goal of treatments (to reach trials) by 2020 and cures by 2025. Now we know that by end of 2020 Marinus will have finished their trial, we will have had at least 4 trials and collectively they will have treated at least 150 kids with CDD that will be able to benefit from these treatments. And I personally believe that by 2025, we will have multiple cures (gene therapies and similar) in advanced clinical trials, possibly even some approved.
If you can imagine it, you can achieve it.
I hope you enjoyed this summary! let me know your thoughts in the comments. Here is my article on the 2018 Forum meeting.
Ana Mingorance, PhD
Disclaimer: These are my own impressions from the presentations that I was most interested in as a scientist and patient advocate, and not an official text about the Forum by the Loulou Foundation. I write these texts with the parents of individuals with CDD in mind, so excuse also my lack of technical accuracy in parts ;-)
Dravet syndrome drug development pipeline review 2019
The 2019 Dravet Syndrome Pipeline and Opportunities Review provides a review and analysis of 12 drug candidates in development for the treatment of Dravet syndrome, including 11 products that have received orphan drug designations. The Report includes the most recent updates on programs from GW Pharmaceuticals (Epidiolex / Epidyolex), Zogenix (Fintepla, ZX008), Biocodex (stiripentol) , Ovid Therapeutics (Soticlestat, OV935, TAK-935), Takeda Pharmaceutical, Supernus Pharmaceuticals (SPN-817, Huperzine), Xeris Pharmaceutical (diazepam), Epygenix Therapeutics (EPK-100, -200 and -300), NeuroCycle Therapeutics (NCT10015), PTC Therapeutics (ataluren), Stoke Therapeutics (STK-001), Encoded Therapeutics and OPKO Health (OPK88001, CUR-1915).
It has been a year since we released the 2018 Dravet Syndrome Pipeline and Opportunities Review, a market research publication that provides an overview of the global therapeutic landscape of Dravet syndrome.
In the last 12 months the pipeline has changed by, among others, the approval by the FDA of Diacomit (stiripentol), the CHMP positive opinion on Epidyolex (cannabidiol) approval, the IPO from Stoke Therapeutics and the arrival of a new company pursuing a gene therapy approach for Dravet syndrome (Encoded Therapeutics).
The 2019 Dravet Syndrome Pipeline and Opportunities Review provides a review and analysis of 12 drug candidates in development for the treatment of Dravet syndrome, including 11 products that have received orphan drug designations.
The Report includes the most recent updates on programs from GW Pharmaceuticals (Epidiolex / Epidyolex), Zogenix (Fintepla, ZX008), Biocodex (stiripentol) , Ovid Therapeutics (Soticlestat, OV935, TAK-935), Takeda Pharmaceutical, Supernus Pharmaceuticals (SPN-817, Huperzine), Xeris Pharmaceutical (diazepam), Epygenix Therapeutics (EPK-100, -200 and -300), NeuroCycle Therapeutics (NCT10015), PTC Therapeutics (ataluren), Stoke Therapeutics (STK-001), Encoded Therapeutics and OPKO Health (OPK88001, CUR-1915).
The 2019 Dravet Syndrome Pipeline and Opportunities Review also includes an analysis of the competitive landscape, unmet needs, and evaluates current and future opportunities of the Dravet syndrome market.
The report is now available in this site.
What the CDKL5 Deficiency community can teach us about patient centricity
I used to say that at the patient communities “we set the agenda”. It turns out we didn’t, we were borrowing the agenda from scientific meetings. The 2019 CDKL5 Alliance International Research and Family Conference redefined what a patient-centered conference truly is. In this article I summarise the elements that make a meeting truly patient-centered.
The first time that I attended a meeting where scientists and patients shared the room was about 12 years ago. It was a small conference on spinal cord injury, and I was an academic postdoc. I remember a man with spinal cord injury, who took the stage and addressed the scientists in the audience. He said “some of you see the glass as half full, and some of you see it half empty. I am here today to ask you that regardless of where you stand on the argument, just grab that glass and do something about it”.
That was my first exposure to an impatient patient.
Some years later I was a scientist at a pharmaceutical company, and we were asked to present an update on our projects to a panel that included scientists, physicians, investors and also some patients. As they all introduced themselves going around the table with the usual line “I’m Dr X and have a PhD in Pharmacology and blah blah”, they reached the man with Parkinson’s disease. He said “I’m Mr X and 6 years ago I was diagnosed with Parkinson’s disease. I feel I’ve earned multiple PhDs on Parkinson’s by now”. He was very right, he was the most knowledge person in that panel.
That was my first exposure to a patient expert, who knew more about a disease than the biotech investors and the physicians sitting next to him.
Last weekend I attended a Research and Family meeting organized by a rare disease patient organization. As a rare disease scientist and patient advocate, I have attended many of these meetings, yet this was my first exposure to a real patient-centric conference. Anything else I saw before, in those 12 years, was a conference that included patients, often organized by patients, but a complete different beast. Let me explain what I mean by this as I review the 2019 CDKL5 Alliance International Research and Family Conference.
2019 CDKL5 Alliance International Research and Family Conference
In June 22nd and 23rd 2019, the CDKL5 Deficiency Disorder (CDD) patient community got together in Edinburgh, UK, for a joint conference with families, clinicians, scientists and pharmaceutical companies.
This one is, for me, a very special patient community. A decade ago, most families with a child diagnosed with CDD were told it was an ultra-rare disease and that they were the only one in their country, or one of a handful at most. Today we know that the disease affects tens of thousands of people, and there is an international patient Alliance that includes 17 national patient organizations and a few other parent-led groups. I’ve been part of this community for the past year and a half since I joined the Loulou Foundation.
There were families from most countries in the Alliance present at the Conference, including many bringing their children with them. Scientists, clinicians and company representatives were outnumbered by the families.
It is only while attending this meeting that I realized that all other patient-organized conferences that I had attended followed one of two formats:
Internal (one country) patient organization meetings, where the focus is the organization itself (accounts, the projects that they finance, priorities)
Broader meetings where clinicians offer updates on clinical trials and scientists offer updates on research to an audience of patient families.
This latter type is the most common. Clinicians and scientists often, but not always, dumb down their explanations so that the families can follow, but other than that it is exactly the same thing that you would expect from a scientific meeting. In many cases the agenda is shaped by the medical or scientific advisors from the patient groups. In other cases, it is designed by the patient organization, but it is still very much inspired in the way scientific meetings are run.
Carol-Anne Partridge, CDKL5 UK
But not the latest CDKL5 Alliance meeting…
The program of this conference was designed by two exceptional people. One is Carol-Anne Partridge, Chair of the host organization, CDKL5 UK, and new Chair of the International CDKL5 Alliance. The outgoing Chair of the Alliance, Rick Upp, introduced Carol-Anne as “one of the 5 Fundamental Forces of Nature” and he is very right. Her stubbornness and determination mean that a meeting organized by CDKL5 UK could not be a conventional meeting.
And Carol-Anne met her perfect match in Philipp von Gallwitz, also a rare disease dad. Philipp is a former pharmaceutical industry professional who now specializes in bridging the patient world and the pharma world. Together they made a program where the focus is on the families attending the meeting, not on the science and clinical presentations.
Once of the scientists in the room described the conference as “celebrating a birthday, or the New Year”. That is how special it was.
Elements of a patient-centric conference
1. The meeting should have a clear mission which is not to update families on what scientists and physicians are doing
The first page of the conference agenda made it clear that the meeting had a specific purpose, and not just to get updates for the families. It read:
“ The main objective is to support families, physicians and industry get ready for clinical trials. Unless otherwise indicated, all information and discussions shall be such that families can understand and contribute. All sessions shall clarify why the presented content is important for families and what they can do in practice to support. Conference moderation will strive to finish all sessions with specific actions and next steps”
The purpose of the conference was to work together towards clinical trial readiness, with patients as active members, not passive listeners. We had to come out of that weekend with a list of clear actions and make the most out of the time we were all together. And so we did.
2. The meeting should start and conclude with families on stage
Right after the host welcomed everyone to the meeting, a mum of a child with CDD explained their story. It was a difficult story for everybody, since she had lost her little boy. It also reminded all why we were in the room: she explained how she shared other families’ pain as if it was her own, and also shared all of their happy moments. That helped us all remember that we are a community and why we were there.
The last speaker of the conference was a grandfather. He was also a great speaker, and helped us see the impact of diseases even beyond the parents and siblings. As in a good speech, opening and concluding the meeting with a common element helped bookend the conference and close the circle around patients.
3. Prepare your presenters to communicate what is important
Philipp made sure that all presenters were briefed about the mission of the meeting and to coach them and review their presentations prior to the conference. Scientists are often used to giving talks where they want to impress other scientists with their hard work and knowledge. Philipp made sure they knew that despite having more scientists in the room, the target audience were the families and the presentations had to clearly explain the medical problem that they were researching, what they found, what it means to the patients and how can families or the community help them. And to do all this in simple terms.
Asking them to simplify the language was key for the meeting success. For example you might love the word “microtubules”, but it works a lot better if you can talk about “the highways along the neurons” as well as the traffic consequences of the mutation.
I sometimes find that families are better at explaining medicine than we (scientists) are. A mum explained to me Cortical Visual Impairment (or cortical blindness), a visual problem in CDD kids due to neuronal problems, not eye problems, as “it is as if the TV works perfectly and the problem is that it is plugged into a non-functioning outlet”. I will remember that one.
4. Use the meeting to build the future: every presentation, panel or discussion should focus on how the patient community can advance the field together with the other stakeholders
Often patient conferences are dominated by scientists and clinicians, with patient families becoming the listener and observer as the professionals explain them what they have been doing “for the patients”. This conference was designed to be different.
Every speaker was instructed to explain to the families what they might need from them: clinical data, blood samples, funding, feedback on their medical scale….
We had multiple sessions where we discussed how the patient community can contribute to advancing the field and how to work together. For example: how can we ensure that even basic research is relevant to patients, how can the patient community provide sufficient support to researchers and physicians, how can we involve families better in trial design and recruitment, etc
Every session was targeted towards identifying practical relevant priorities, actions, quick wins, strategies…. That focus on actionable elements means we all went home with an entire notebook of actions and that the CDKL5 Alliance will have a very busy year!
5. Maximize the time and opportunities to listen to the patients / families
At the 2019 CDKL5 Alliance International Research and Family Conference there was no audience. There were only participants.
Everybody in that room had multiple opportunities to contribute, whether they were patient families or scientists or company representatives.
Patient Experience Workshop
We had a panel session where the entire room was the panel. We discussed what researchers need from families and what families need from researchers. We learned wonderful things that I’m sure wouldn’t have come up in a more structured meeting. For example I learned that because there are only 6 patient families in Ireland, they felt they were not enough to come together and organize an annual meeting, but ever since there was also a research lab in Dublin dedicated to CDKL5 the families felt it was the perfect reason to come together with the scientists and host their first annual meeting. The academic lab helped create the momentum that the small patient community needed.
We had a World Café, where all of the participants divided in 4 groups an discussed the main challenges, opportunities and role for patients and professionals in research, development, clinical care and clinical trials. Every single person participated in a brainstorming on each of the 4 topics, and contributed to a list of action items and a roadmap.
We had a patient experience workshop where all families were invited to provide their testimonies on the symptoms and impacts of living with CDD and their hopes and perspectives on treatments. It was shaped as a mini-PFDD meeting, where we only listen to the caregivers in a facilitated discussion, and it was the most amazing experience I’ve had a patient conference. Many of the families also told me how it had been the highlight of the day, and I will try to incorporate similar sessions in future patient conferences. For the industry professionals present at the meeting it was also an exceptional opportunity to learn how to see the disease through the eyes of the patient families, and how different it can be from the simpler clinical description of the disease in medical publications.
There was truly no audience at this conference, only participants.
6. Incorporate the community leadership
“There are 5 Fundamental Forces of Nature: gravity, weak nuclear force, strong nuclear force, one that I can never remember, and Carol-Anne Partridge” . Rick Upp, about Carol-Anne Partridge
The CDKL5 Alliance also hosted a closed meeting with all of the representatives from the national CDD patient organizations. Every year, the country hosting the international conference becomes the next Chair of the Alliance. So just as the UK brexits away from everybody else, CDKL5 UK takes the leadership of this exceptional patient community from the previous US Chair.
The mission of the Alliance is to collaborate and support each other, identify more patients, set up new clinical trial sites and centers of excellence, and overall to support the medical and research community as we progress towards new treatments for the disease. Running an annual international meeting the size of this one is not easy, but it is an exceptional opportunity to bring all of the organizations together under the same roof and keep it together and productive.
For the CDKL5 Alliance, the new year started on June 23rd, and I look forward to supporting them in these next 12 months.
Final notes
I personally love family conferences. I love to get to meet many new families and learn from them. I loved meeting Eric, who can’t yet speak or hold his head up but seriously likes traditional Scottish music, and to meet families from my home country. I get frustrated in these conferences at the slow pace of drug development, but I also get much more energized and inspired so the net effect is very positive.
I used to say that at the patient communities “we set the agenda”. It turns out we didn’t, we were borrowing the agenda from scientific meetings.
Thank you Carol-Anne and Philipp for truly setting up the agenda, and redefining what a patient-centric conference is truly about.
Let me know your thoughts in the comments,
Ana Mingorance, PhD
Image credits: some are my pictures, some are pictures other participants took and shared in social media
Clinical trials in CDKL5 Deficiency Disorder – 2Q 2019
There are currently 4 clinical trials ongoing or about to start in CDKL5 Deficiency Disorder: ataluren, ganaxolone, TAK-935 and fenfluramine. This article is a summary of where we are with clinical trials for CDKL5 Deficiency Disorder for families and other interested readers including what we know about these four drugs, their efficacy, at which level of clinical development they are at, and where can you learn more about these trials.
Some rare diseases can go for many years without much research on them. Other rare diseases, however, attract so much attention from scientists and companies that in a couple of years can make the progress that would have otherwise taken decades.
This is the case of CDKL5 Deficiency Disorder (CDD), a monogenetic rare disease that affects brain development and causes a very severe epilepsy with hundreds of seizures a month. I also reviewed all of the news on CDD in my review of the last CDKL5 Forum meeting HERE (October 2018).
This article is a summary of where we are with clinical trials for CDKL5 Deficiency Disorder for families and other interested readers.
FIRST, A NOTE ABOUT CLINICAL TRIALS
There are currently 4 clinical trials ongoing or about to start in CDD.
If you are coming from the patient side, and not from the medical community, you have probably heard about clinical trials being always divided in three stages:
Phase 1 trials: Small trial (study) in healthy adult volunteers, not in patients. The purpose of this phase is to determine the safety of the drug, as well as to explore different doses of the drug and measure the biodistribution (how soon you eliminate it, how it breaks down, does it accumulate… etc)
Phase 2 trials: Also known as “pilot” trials. Small trials in real patients with the disease that the drug intents to treat. This phase is mainly intended for determining safety, and also to pick a sign of efficacy. There are usually not enough patients to be sure that the drug works, but it enables companies and regulators to decide to move on to the next phase if the data looks good.
Phase 3 trials: Also known as “pivotal” trials. These are large confirmatory studies, in patients, with a placebo-controlled group (to serve as a control of what the normal change in the disease would have been in the absence of the drug, everything else remaining the same). You often need two of these trials for approval. Depending on the disease, it could be over a thousand patients per group. Other times it is many hundreds. Because of the need of finding so many patients it can take years to complete.
This is the default design, but there are exceptions to it. One exception is that when diseases are rare, you often need fewer patients, and might only need one pivotal trial to show efficacy and get a drug approved. This means a company can go from starting clinical trials to approval in 4 years, as GW Pharma recently did with Epidiolex for Dravet and Lennox-Gastaut syndromes, as opposed to 10+ years on non-orphan diseases!
Another exception is when the drug being tested has already gone through a Phase 1 evaluation in the past when being considered for other diseases, so once the company that owns the drug shows an interest in your disease they can move straight into Phase 2 (pilot) trials.
Both exceptions are true for CDD, so as you will see below our timelines are much faster than the usual length of Phase 1 + Phase 2 + Phase 3 trials that you will find described in most on-line materials. Also, our pilot (Phase 2) trials need less than 20 patients, and the first drug that has reached the pivotal (Phase 3) stage only needs one trial with 70 patients. This makes it all a bit more doable and a lot faster.
One important note about CDD trials is that all of them add the experimental drug or the placebo treatment to the other medications that the patient is already taking for a duration of 12 to 17 weeks. This means that no participant will ever find themselves in a “no treatment” trial group if receiving placebo, they will simply start adding the actual experimental treatment to their usual medications later. Some of the clinical trials do not even include placebo group.
So let’s jump into the update: which are these four drugs, what do we know about their efficacy, and where can you learn more about these trials or what comes after them.
CDKL5 DEFICIENCY DISORDER CLINICAL TRIALS REVIEW
From the drug that first started clinical trials in CDD to the one that is about to start, these are the four clinical trials for CDD that you should know about:
#1 ATALUREN – PTC THERAPEUTICS
Ataluren is a drug approved in Europe for the treatment of a subset of patients with Duchenne Muscular Dystrophy and marketed under the name of Translarna. It is currently completing a placebo-controlled Phase 2 (pilot) study at NYU Langone Medical Center in children with CDKL5 Deficiency Disorder caused by non-sense mutations.
How does this drug work?
The brand name of ataluren is “Translarna” because it facilitates translationof RNA. I like how clever the name is. What it does is to target specifically a type of mutations known as non-sense mutations, which cause a premature stop in the gene sequence. These mutations appear in many different genes. That is why we can test this drug in diseases other than Duchenne, such as CDD and Dravet syndrome (the second disease evaluated in the same clinical trial). What ataluren does is to make the cell read through that premature stop and complete the protein that is otherwise missing in that disease.
Is there any previous clinical experience with this drug?
Yes! Ataluren is already approved for Duchenne, so the active dose and the safety profile are known. There is still no efficacy data in patients with neurodevelopmental diseases or epilepsy because these CDD and Dravet syndrome trials are still ongoing. One important difference with Duchenne is that CDD and Dravet are diseases of the brain, not the muscles, so it is possible that the drug doesn’t get well-enough into the neurons to work in these neurodevelopmental diseases. We will have to wait for the trial results to know that.
How is the clinical trial in CDD?
The trial is a pilot study, involving 9 patients with CDD that all go through some months in treatment and some months in placebo. This is called a “crossover” design, where some patients start in the drug and are then crossed over to placebo, and others start in placebo and are then crossed over to drug. This way, none of the patients in the study has to be only in placebo, and it also means that each patient is their own control.
The trial measures epilepsy as the main symptom for improvement. They also track cognitive function and quality of life as additional potential areas of improvement.
What would be the next steps for approval?
This trial is only taking place at one hospital, NYU Langone Medical Center, because it is what is known as an “investigator-initiated study”. Investigator-initiated means that the company that owns ataluren was not who decided to start this trial, but the investigator (the clinician) from NYU approached the company and asked them to let them run this clinical trial at their hospital.
Because it is a small pilot trial, the resulting data will not be sufficient for requesting approval of ataluren for treating CDD. So first the drug results need to be published, it has now completed recruitment but the data collection and publication are not yet completed. And after that, if the data is positive, PTC would need to run a Phase 3 pivotal trial. So the next step to this clinical trial is another (larger) clinical trial if the results are positive.
Where can I find more information about the trial?
The clinical trial and contact information are here.
#2 GANAXOLONE – MARINUS PHARMACEUTICALS
Ganaxolone is a drug currently in Phase 3 (pivotal) trials in CDD. It is also in clinical trials for other neurological diseases. It has not yet been approved for any disease, and if everything goes well it is likely to become the first drug to be ever approved for the treatment of CDD since it is the most advanced one.
How does this drug work?
You might be familiar with medications like Valium and Xanax. They belong to a class of drugs, benzodiazepines, that are used for anxiety, insomnia and muscle relaxation among other uses. Some drugs in this class, like clobazam (Onfi) are even used for treating epilepsy. These drugs all enhance the activity of a type of brain receptors called GABA receptors and the result is “brain relaxation”. The brain of people with CDD has an excess of neuronal activity, so drugs that enhance or facilitate GABA receptor function can help reduce excessive activity and minimize some of the symptoms.
Ganaxolone binds to the GABA receptor and enhances their activity in a different way to how benzodiazepines work, so it is thought to achieve the “brain relaxation” with slightly different properties. It is also known that in some epilepsy syndromes, patients have low blood levels of a ganaxolone-like endogenous body hormone, so part of the efficacy of ganaxolone could be due to it helping correct this deficiency.
Is there any previous clinical experience with this drug?
Yes. Ganaxolone has been in clinical trials for other neurological diseases, like Fragile X syndrome and partial onset (focal) seizures in adults. More importantly, there has been a Phase 2 (pilot) trial in people with PCDH19 epilepsy and CDD that sowed it had efficacy on both patient populations when looking at their epilepsy. Because ganaxolone was also safe, it has now been progressed to the final Phase 3 (pivotal) studies in PCDH19 epilepsy and in CDD, now in two separate studies.
How is the clinical trial in CDD?
The Phase 3 clinical trial of ganaxolone in CDD is called the Marigold Study and takes place at numerous centers internationally. The trial is currently recruiting for patients. You can find more information about trial sites in the website of the Marigold study.
To enroll in the trial the patient needs to have a mutation in CDKL5, be 2-21 years old, and have at least 16 “major seizures” per month, which includes tonic-clonic seizures and atonic (drop) seizures. They are looking for at least 70 trial participants.
During the trial some of the patients are given ganaxolone while some receive placebo. Neither the families nor the physicians know which group the patient is in. After 17 weeks all patients are offered a chance to take ganaxolone, so if your child is placed in the placebo group it just means they will be starting the actual treatment about 4 months later.
The trial will measure epilepsy as the main symptom for improvement, and will also track improvement in other areas such as attention and behavior.
What would be the next steps for approval?
The Marigold study is a pivotal trial, meaning that it is a final trial. Once the study is completed, Marinus will submit all the documentation to the different regulatory agencies and request the marketing authorization for the treatment of CDD.
Where can I find more information about the trial?
Marinus has created a website specifically for this trial: the Marigold study. You can also find more information as well and some CDD materials for patients at the company website.
#3 – TAK-935 / OV935 – OVID THERAPEUTICS AND TAKEDA PHARMA
TAK-935, also known as OV935 since it is co-developed by Takeda and Ovid, is a drug currently in Phase 2 trials in CDD and other neurodevelopmental syndromes with epilepsy. It is an experimental drug and it is not yet approved for any other disease.
How does this drug work?
As you will remember from some paragraphs before, GABA is an inhibitory substance in the brain, which is why some drugs like ganaxolone enhance the activity of the GABA receptors to reduce brain excessive activity. They enhance brain inhibition. The excitatory substance in the brain is glutamate, and we know that the brains of people with CDD have an excess of neuronal activity in part due to too much glutamate signaling. What TAK-935 does is to reduce this excessive activity by reducing glutamate signaling, therefore bringing brain activity down to healthier levels. It reduces brain excitation.
Is there any previous clinical experience with this drug?
TAK-935 had already been in clinical trials for other neurological diseases although it was never taken all the way to the market. Because of that, the Phase 1 trials were already done. When Ovid and Takeda decided to test TAK-935 in drug-refractory epilepsies they run a Phase 2 (pilot) trial with 18 patients with a variety of rare epilepsy syndromes. Because the safety was good, and the patients experienced a reduction in seizures, the companies decided to progress the drug to further testing, and it is now being studied in four different Phase 2 (pilot) trials: one for Lennox-Gastaut syndrome, one for Dravet syndrome, one for Dup15q syndrome, and one for CDD.
How is the clinical trial in CDD?
The CDD and Dup15q studies are combined under a trial called the ARCADE study. The trial is currently recruiting patients, and is looking for 15 people with CDD ages 2 to 35 with at least 3 motor seizures per month.
Because it is a pilot study, there is no placebo group. All 15 participants will receive the experimental drug on top of their regular baseline medication. Participants will take TAK-935 for 20 weeks (8 weeks bringing up the dose slowly followed by 12 weeks at maintenance levels), and the total trial duration beginning to end is 30 weeks. At the end of this period all participants will be offered to keep taking the drug if they found it to be effective.
The trial will measure epilepsy as the main symptom for improvement, and will also track general improvement.
What would be the next steps for approval?
Because the clinical trial is a Phase 2 (pilot) trial, and not a final pivotal trial, the results of the trial will not be sufficient for the companies to request a marketing authorization. If the trial results are good, then they will have to run one or two Phase 3 (pivotal) clinical trials like the one currently ongoing with ganaxolone, involving many more patients and most likely a placebo group. So the next step to this clinical trial is another (larger) clinical trial if the results are positive.
Where can I find more information about the trial?
You can find more information about this trial in the ARCADE study website.
#4 - FENFLURAMINE – ZOGENIX
Fenfluramine is a drug that was approved many years ago for treating obesity as part of a combination pill. It was later discovered to have efficacy in treating drug-refractory epilepsy in an epilepsy syndrome called Dravet syndrome, and it has now completed all of the clinical trials for Dravet syndrome and is awaiting regulatory review to obtain the marketing authorization. Pilot studies have shown that fenfluramine has efficacy in other syndromes as well, so a pivotal trial is ongoing for Lennox-Gastaut syndrome and the company is interested in evaluating the efficacy of their drug in other syndromes with epilepsy to identify potential new diseases that could benefit from it. One of those, is CDD.
How does this drug work?
While GABA and glutamate are respectively the main inhibitory and excitatory substances in the brain, there are several other substances that are known as “modulatory” because they tweak brain activity in many different ways. One of these is serotonin, and you might be familiar with antidepressants increasing serotonin signaling to stabilize people mood. This is also what fenfluramine does. Fenfluramine enhances serotonin signaling through some of the serotonin receptors, of which they are in total 15 different ones each playing different roles in the brain. It is not clearly known why these serotonin receptors are involved in epilepsy, but the clinical data so far has shown that fenfluramine is a very good anti-epileptic drug, at least in children with Dravet syndrome. Fenfluramine might also bind to other receptors in the brain, this is all still being studied.
Is there any previous clinical experience with this drug?
Yes! The reason why fenfluramine will be studied in CDD is because of the results they have seen with Dravet syndrome. Dravet syndrome is another neurodevelopmental syndrome with epilepsy, and unlike CDD where some of the patients ultimately outgrow their seizures, in Dravet syndrome this doesn’t happen. Two Phase 3 (pivotal) trials with fenfluramine in these children showed that fenfluramine could reduce seizure frequency by more than 70%, and about one in four participants was seizure free or “near seizure free” (about 4 seizures a year). The side effect profile of fenfluramine is similar to other anti-epileptic drugs and it requires extra cardiac monitoring.
How is the clinical trial in CDD?
The trial is a pilot study, involving 10 patients with CDD that all receive the drug because there is no placebo group. The trial has not yet started recruiting, it will take place at NYU Langone Medical Center, and is looking for children with CDD ages 2 to 18 with more than 4 convulsive seizures a month that will receive the drug added to their baseline medication during 14 weeks.
The trial measures epilepsy as the main symptom for improvement. They also track quality of life and general improvement.
What would be the next steps for approval?
This trial is only taking place at one hospital, NYU Langone Medical Center, because it is again an “investigator-initiated study” as explained above for ataluren.
Because it is a small pilot trial, the resulting data will not be sufficient for requesting approval of fenfluramine for treating CDD. So the next step to this clinical trial is another (larger) clinical trial if the results are positive.
Where can I find more information about the trial?
You can find more clinical trial and contact information HERE, and additional information about previous results with fenfluramine in Dravet and Lennox-Gastaut syndromes HERE.
THE FUTURE - IS THERE ANY MORE RESEARCH ONGOING BEYOND THESE TRIALS?
Oh yes! These four are all therapies that were already developed for treating other diseases (most for epilepsy, ataluren for addressing one specific mutation type). This means that they could move really fast into CDD, and start clinical trials right away without needing more research or improvements on them.
But there are other very exciting programs that have been started specifically to address the genetic cause of CDD. These ones will still take a couple of years before they can start clinical trials because they have been started from scratch to be designed for CDD, and that takes time that we didn’t have to wait with the “ready for clinical trials” drugs.
As a reminder of how CDD happens, CDKL5 is both the name of a gene and the protein that it produces. Each protein in the body has a specific function. The CDKL5 protein function is to put a phosphate onto other proteins which is like an on/off switch for those other proteins. This allows CDKL5 to turn on and off many functions of the neurons. Proteins that do this are called enzymes.
We are all born with mutations that were not present in our parents, that is how evolution works, but in most cases these mutations are in non-important regions, or at least are not too damaging. When one of these mutations, however, happens in the CDKL5 gene sequence and either breaks the sequence or gives it the wrong instructions, then that person cannot produce the CDKL5 protein or produces a non-functional version. Without good CDKL5 protein, all of those functions in neurons that needed CDKL5 to put all of the on/off switches in the right configuration are now not functioning properly. This is why the deficiency in CDKL5 is so bad for the brain.
There are two main efforts to fix these problems in patients with CDD. Not to treat their symptoms, but to correct the faulty biology that is causing the symptoms. These are the type of approaches that in the patient community we often call cures, although you will not hear this word from pharmaceutical companies. They prefer to call them disease-modifyingtreatments because they change the disease.
The first approach is gene therapy. If you could give each neuron a new copy of the CDKL5 gene then they will be able to produce the protein and function as a normal cell. There are multiple efforts going on in this area, but I will highlight the program from the company Ultragenyx, who announced last October that they will develop a virus carrying the CDKL5 gene as a gene therapy for CDD.
The second approach is to simply add to the brain the CDKL5 protein. This has been done in the past in other diseases caused by enzyme deficiencies, and are known as Enzyme Replacement Therapies. I would highlight here that the company Amicus has been working on this approach for the last couple of years, trying different approaches to deliver the CDKl5 protein to the brain.
This means that in the next couple of years we will have several clinical trials for CDD that target disease symptoms, and then we will start having clinical trials with therapies that target the cause of the disease. The first group of drugs will reduce the symptoms of the disease and give the patients a chance to acquire more skills faster while they have less seizure burden. The second group of therapies, in particular when used in very young kids, will lead us to a future where children born with mutations in CDKL5 will pretty much be able to grow as if their gene was not mutated. As usual in medicine some trials will fail, but other trials will get started. The important message is that there are so many programs ongoing that the question is not IF we are going to make it to effective therapies, but WHEN.
Let me know if you have some questions on these trials that I didn’t cover in the article!
Ana Mingorance, PhD